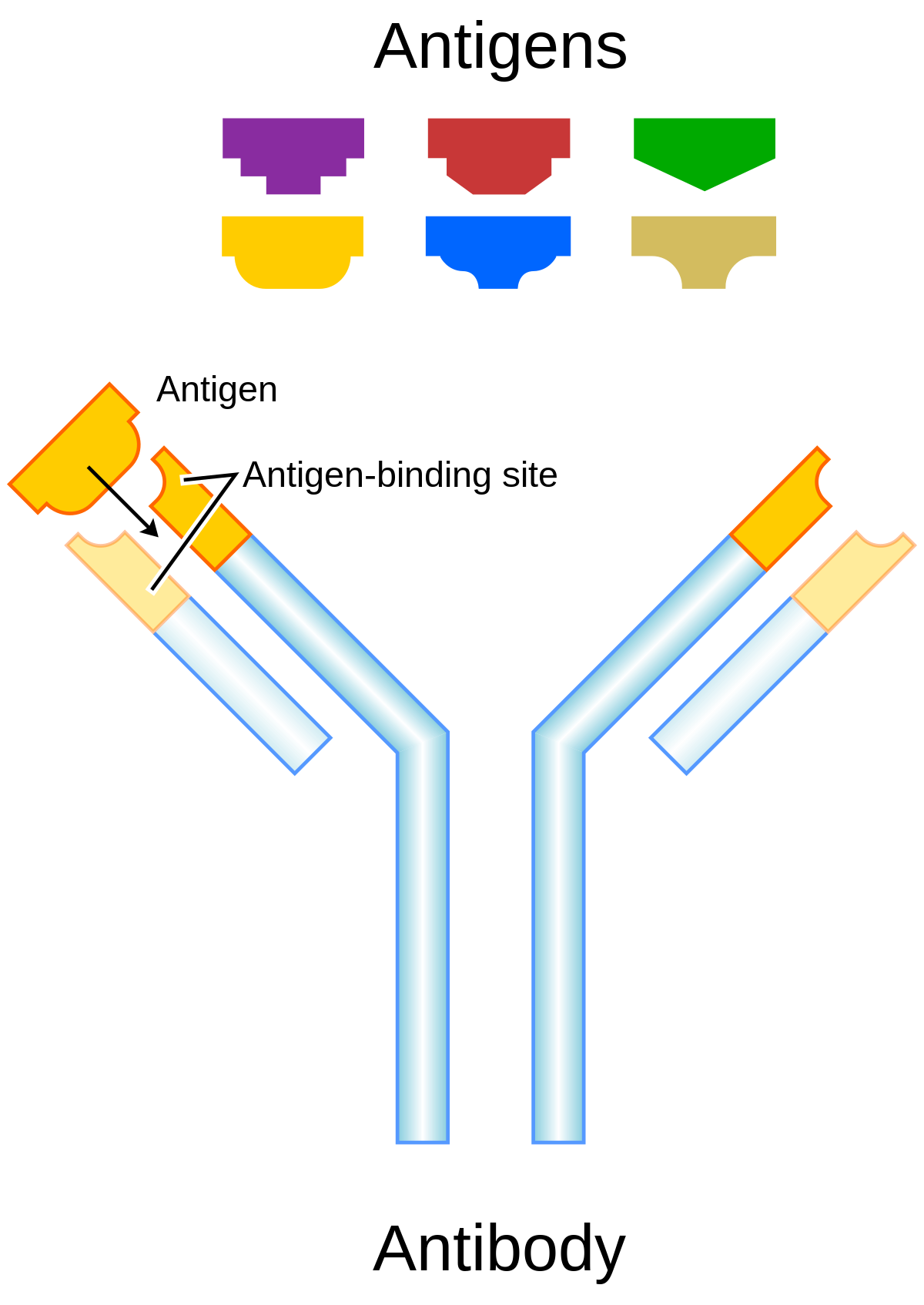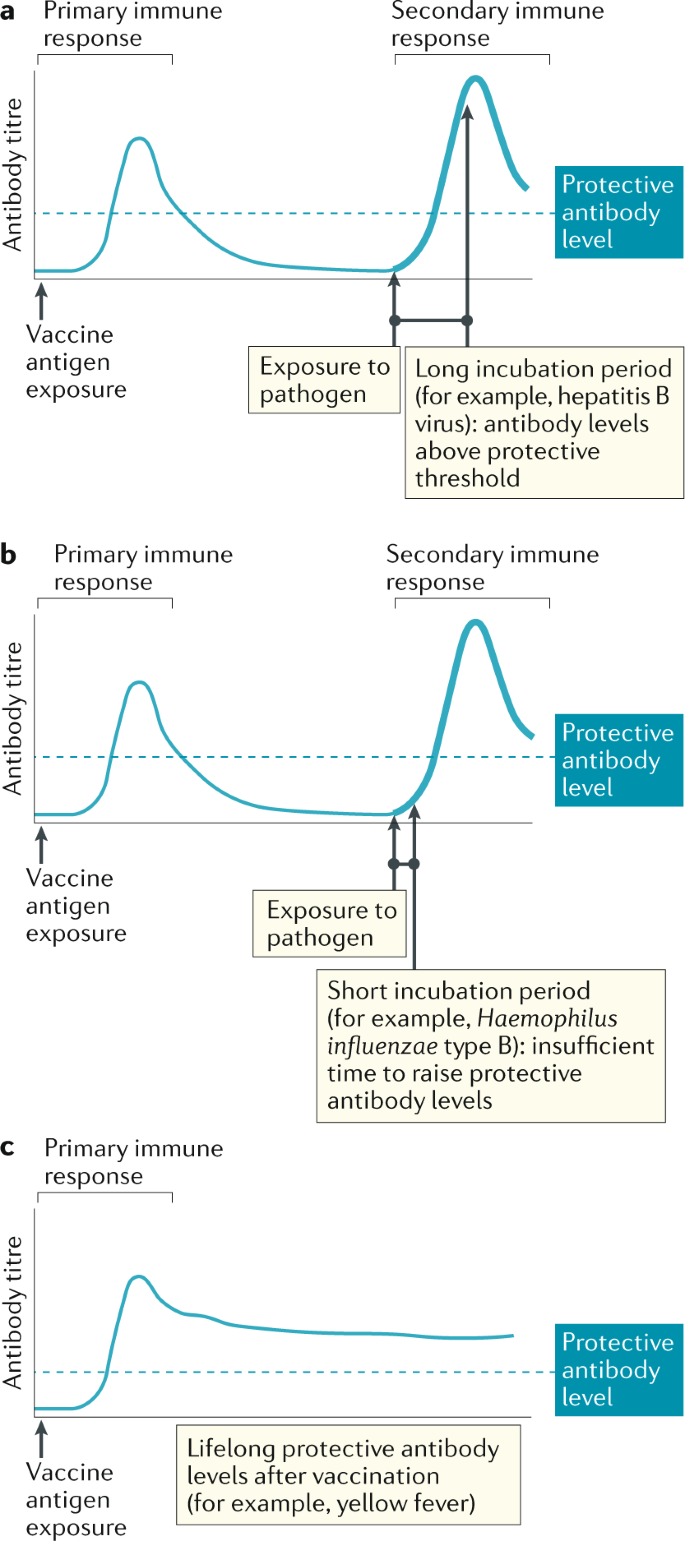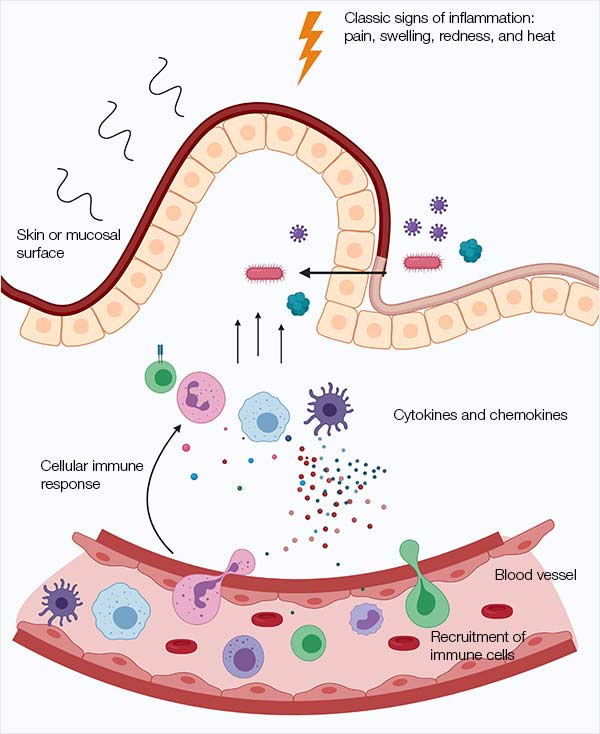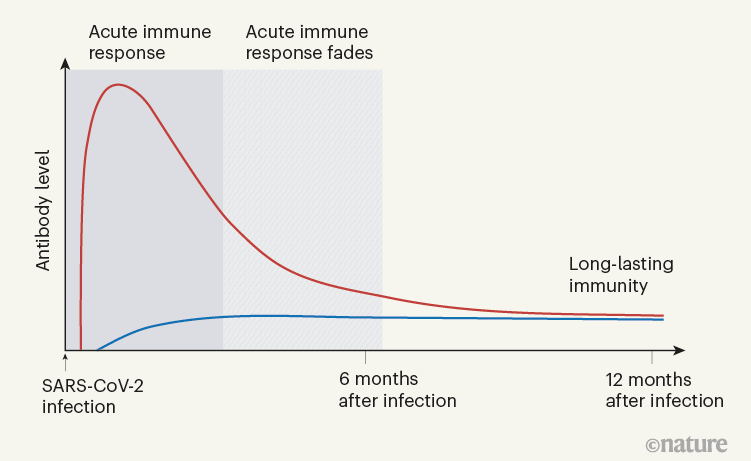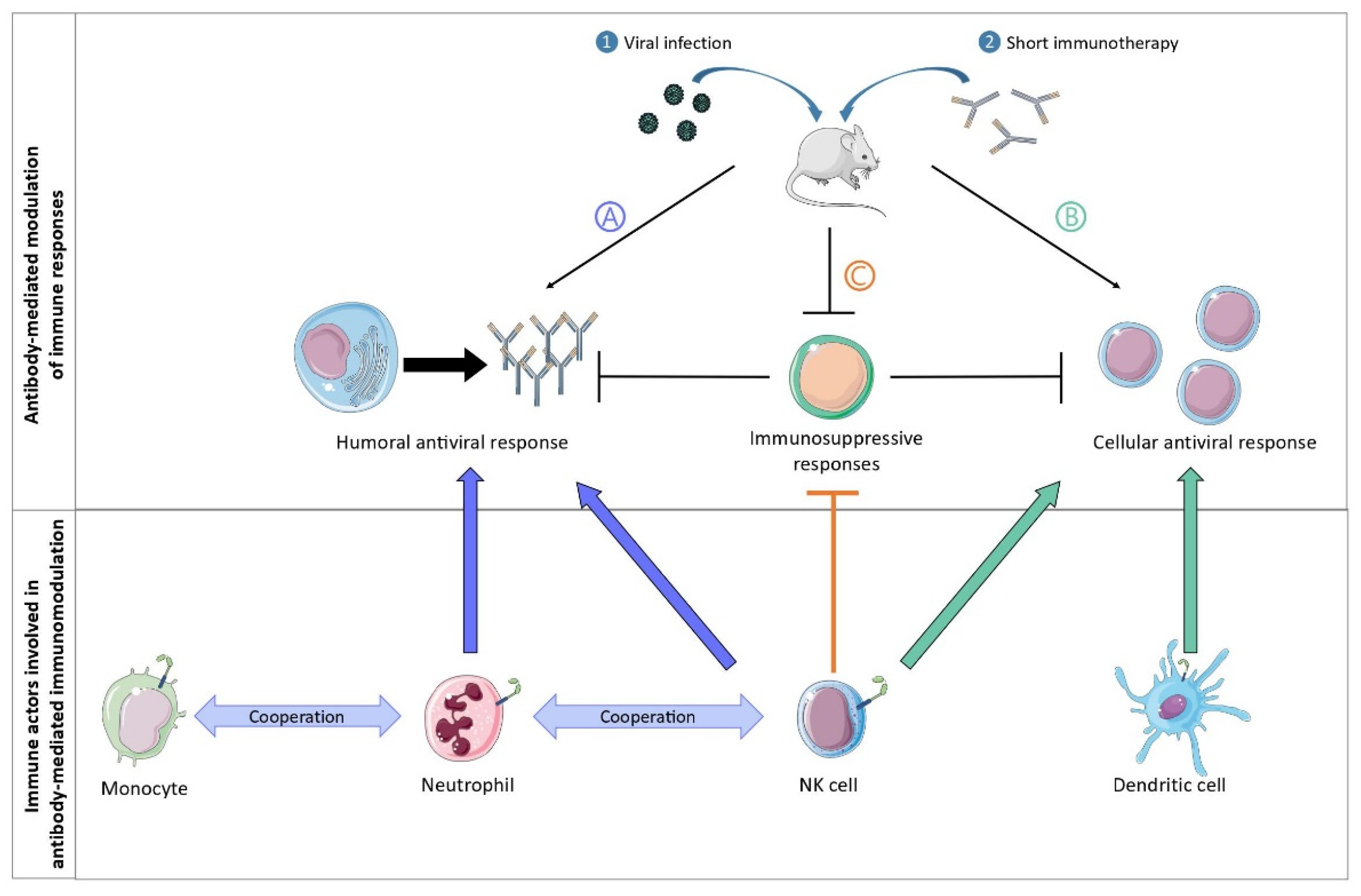
Antibodies | Free Full-Text | Fc-Dependent Immunomodulation Induced by Antiviral Therapeutic Antibodies: New Perspectives for Eliciting Protective Immune Responses

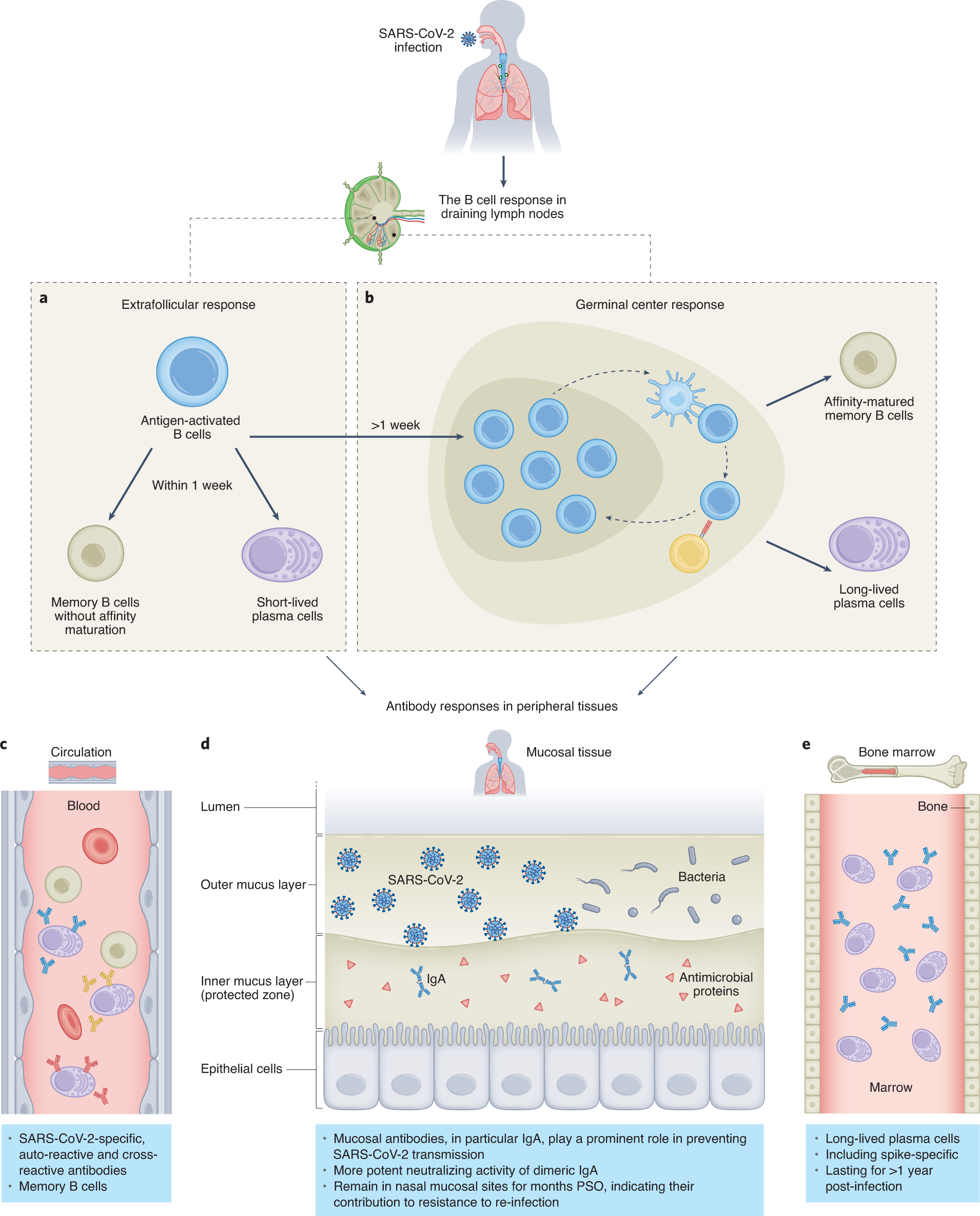
Defining the features and duration of antibody responses to SARS-CoV-2 infection associated with disease severity and outcome | Science Immunology
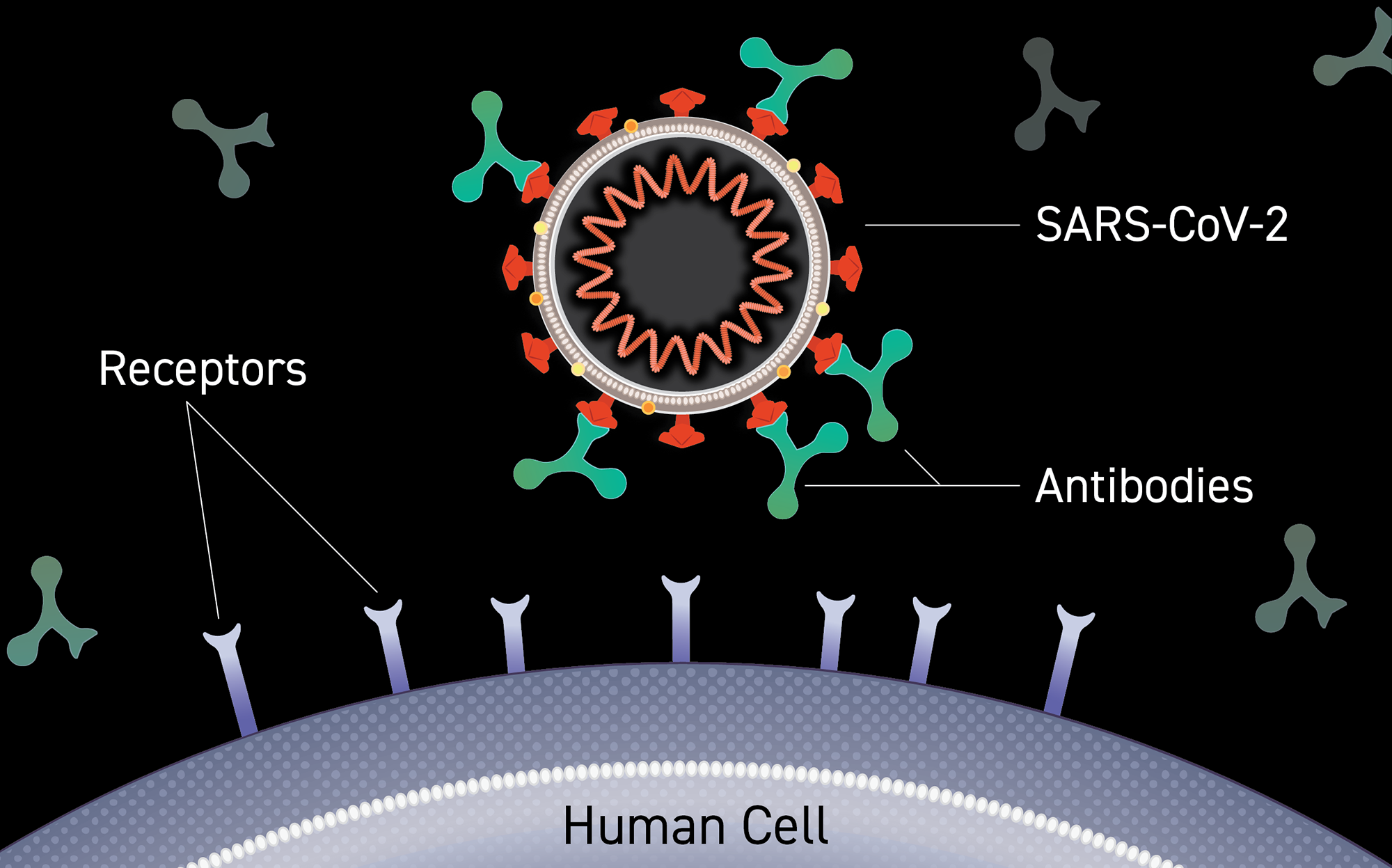
Clinical trials of monoclonal antibodies to prevent COVID-19 now enrolling | National Institutes of Health (NIH)

Prevalence and duration of detectable SARS-CoV-2 nucleocapsid antibodies in staff and residents of long-term care facilities over the first year of the pandemic (VIVALDI study): prospective cohort study in England - The
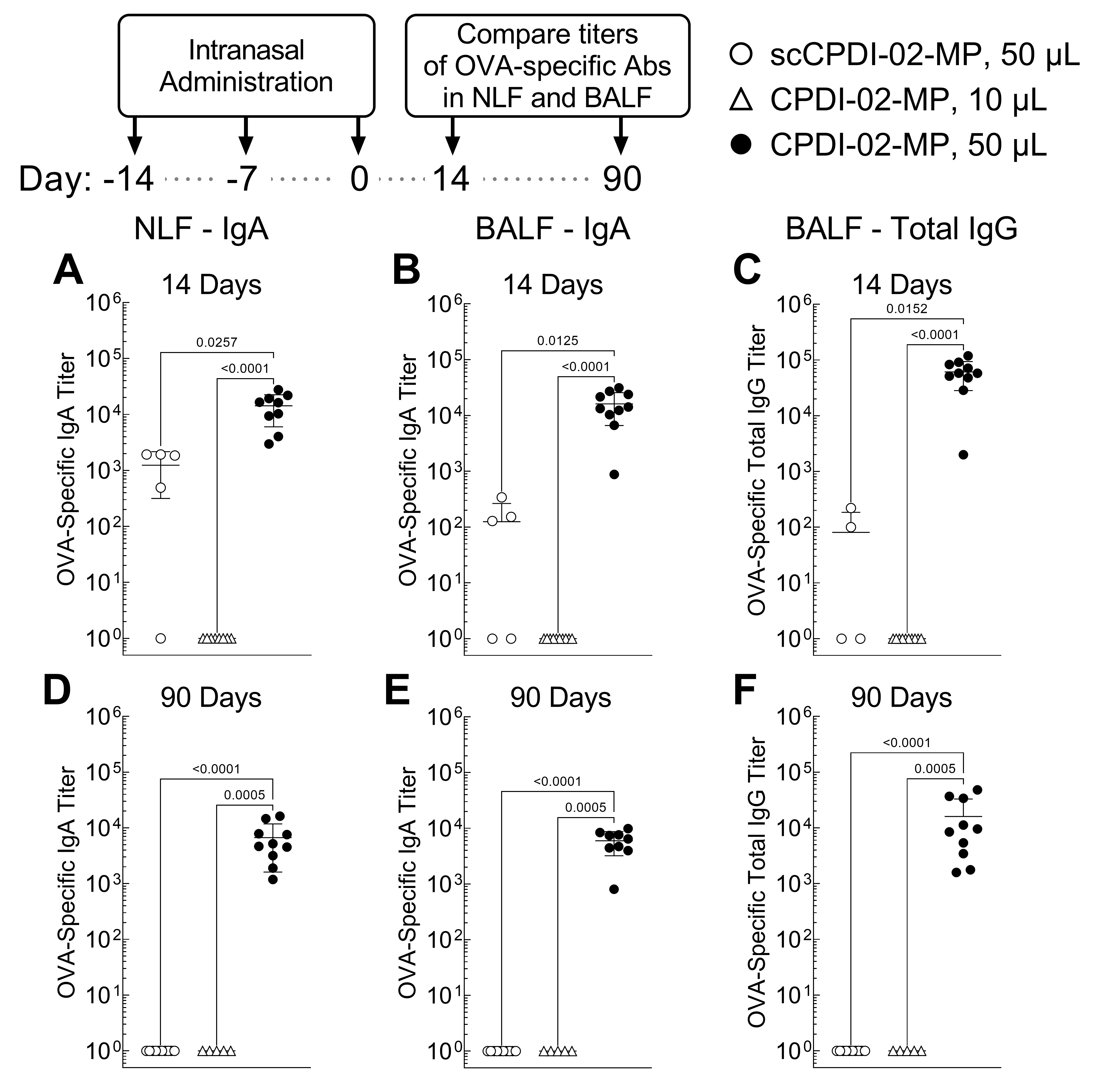
Pharmaceutics | Free Full-Text | Surface Modification of Biodegradable Microparticles with the Novel Host-Derived Immunostimulant CPDI-02 Significantly Increases Short-Term and Long-Term Mucosal and Systemic Antibodies against Encapsulated Protein ...
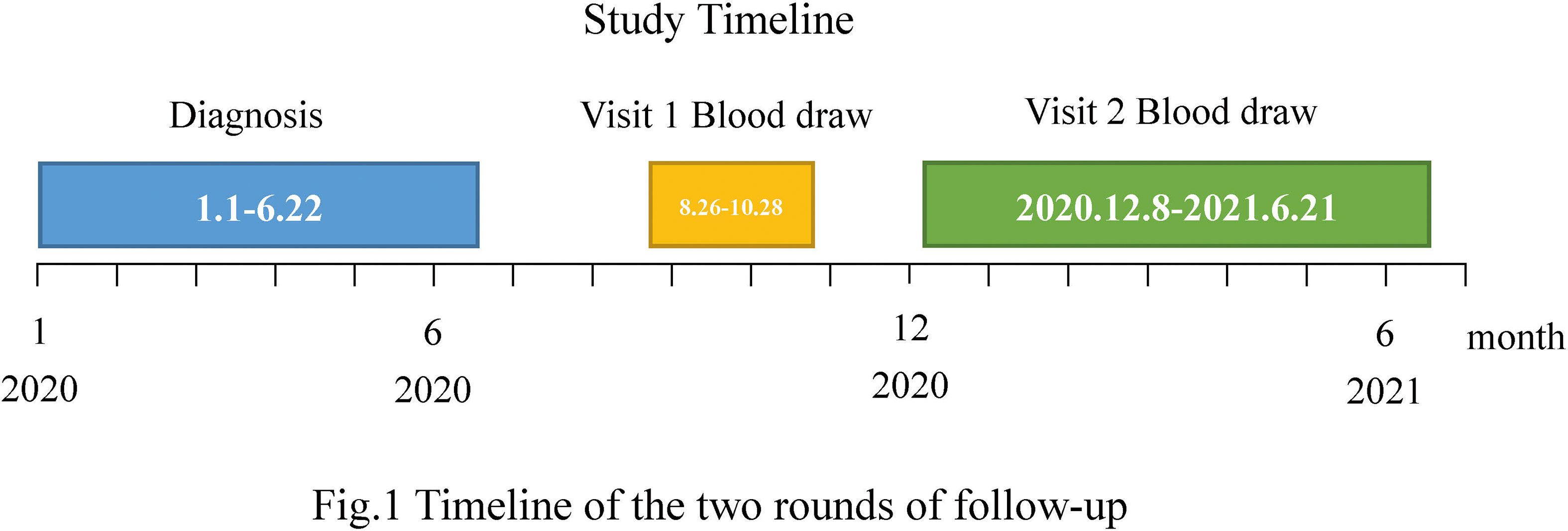
Frontiers | Long-Term Kinetics of SARS-CoV-2 Antibodies and Impact of Inactivated Vaccine on SARS-CoV-2 Antibodies Based on a COVID-19 Patients Cohort
Long-term treatment with anti-CD20 monoclonal antibodies is untenable because of risk: YES - Chiara Zecca, Claudio Gobbi, 2022

Antibodies | Free Full-Text | Long-Term Immunity and Antibody Response: Challenges for Developing Efficient COVID-19 Vaccines

Single-Administration Long-Acting Microarray Patch with Ultrahigh Loading Capacity and Multiple Releases of Thermally Stable Antibodies | Molecular Pharmaceutics

Robust long-term immunity to SARS-CoV-2 in patients recovered from severe COVID-19 after interleukin-6 blockade - eBioMedicine