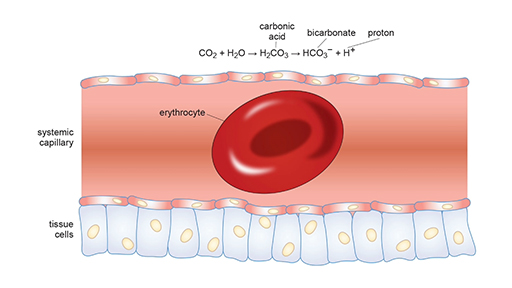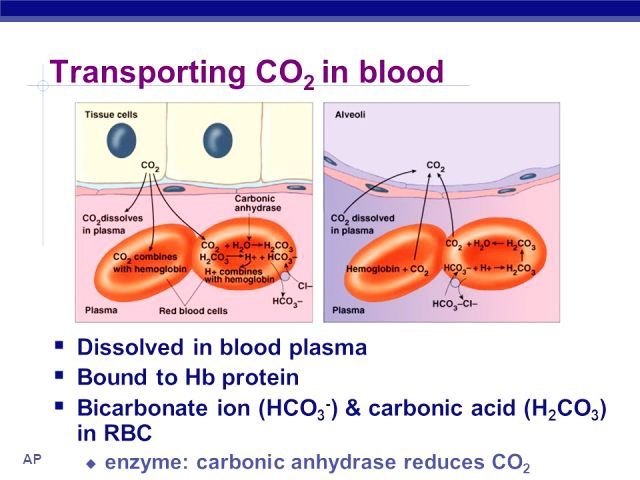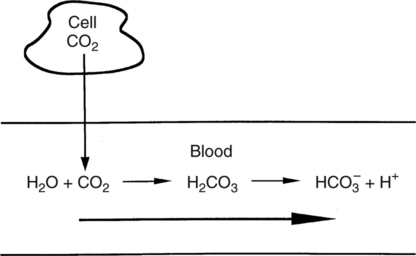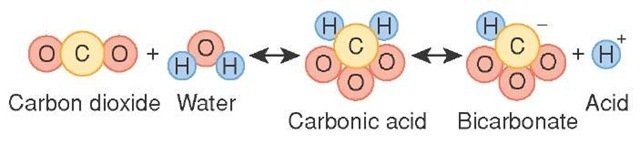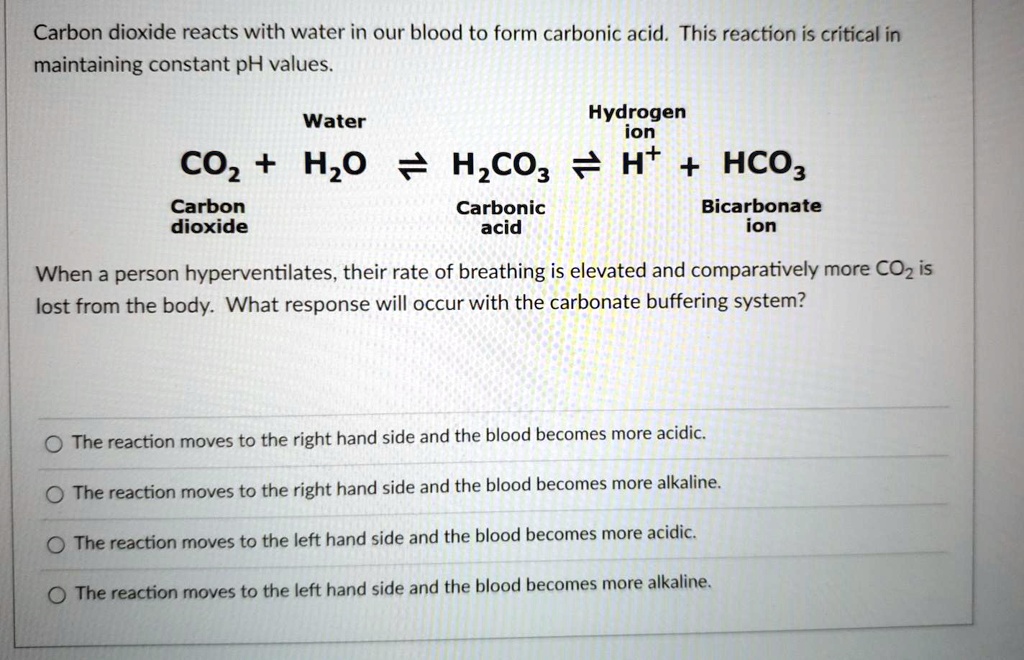
SOLVED: Carbon dioxide reacts with water in our blood to form carbonic acid. This reaction is critical in maintaining constant pH values Water Hydrogen ion HzCOz H+ HCO3 Carbonic Bicarbonate acid ion

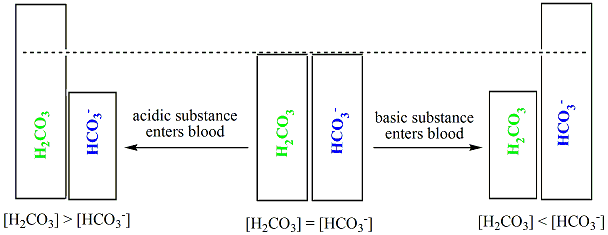
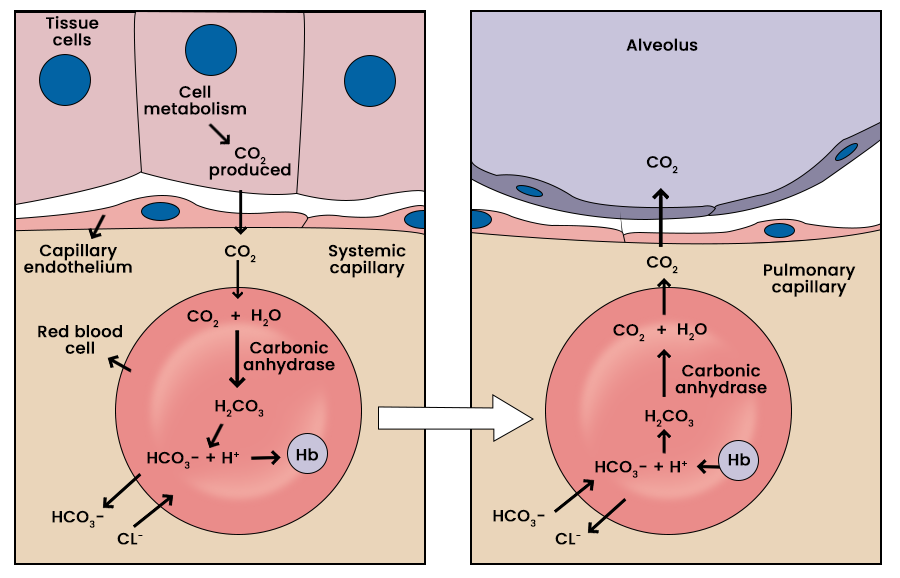
During normal respiration carbonic acid (H2CO3) and bicarbonate (HCO3-) help to regulate the amount of H+ ions in the blood. CO2 in the blood is readily converted to carbonic acid and most
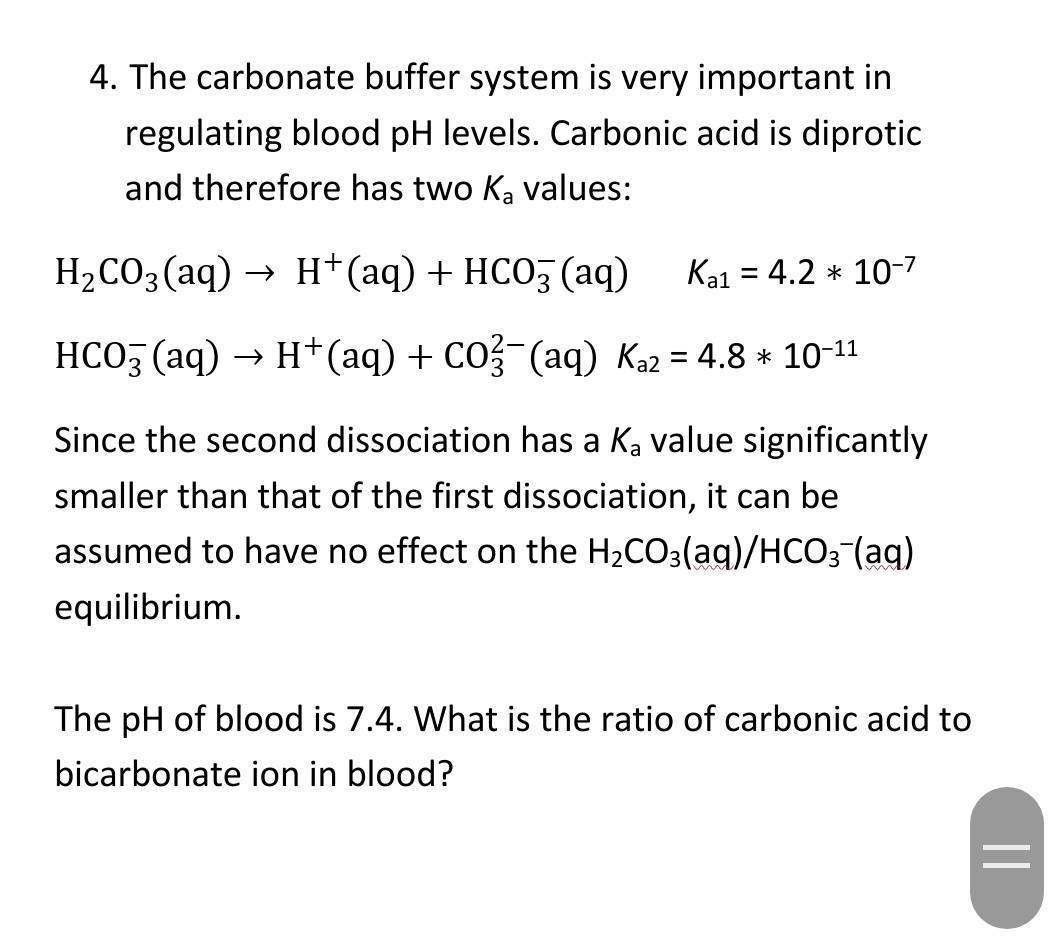
![SOLVED: A buffer system in the blood consists of the bicarbonate ion, HCO3-, and carbonic acid, H2CO3. What value of the ratio [HCO3-]/[H2CO3] would be required to maintain blood at the average SOLVED: A buffer system in the blood consists of the bicarbonate ion, HCO3-, and carbonic acid, H2CO3. What value of the ratio [HCO3-]/[H2CO3] would be required to maintain blood at the average](https://cdn.numerade.com/ask_previews/66f77e37-e822-4338-88cc-54bfa1896a66_large.jpg)