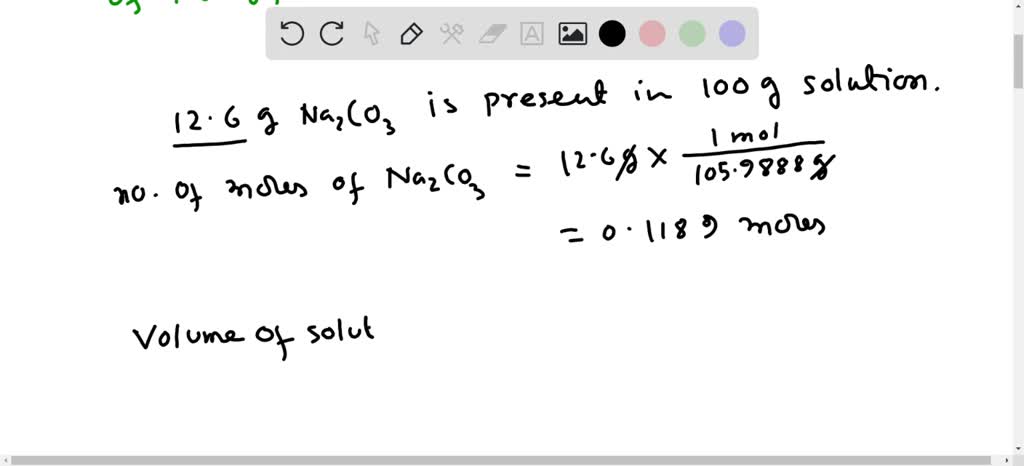Density, Viscosity, and Surface Tension of Sodium Carbonate + Sodium Bicarbonate Buffer Solutions in the Presence of Glycerine, Glucose, and Sucrose from 25 to 40 °C | Journal of Chemical & Engineering Data

Density, Viscosity, and Surface Tension of Sodium Carbonate + Sodium Bicarbonate Buffer Solutions in the Presence of Glycerine, Glucose, and Sucrose from 25 to 40 °C | Journal of Chemical & Engineering Data

A sample of pure sodium carbonate 0.318 g is dissolved in water and titrated with HCl solution. A volume of 60 mL is required to reach the methyl orange end point. Calculate

Influence of solution temperature, sodium carbonate concentration, and... | Download Scientific Diagram

Density, Viscosity, and Surface Tension of Sodium Carbonate + Sodium Bicarbonate Buffer Solutions in the Presence of Glycerine, Glucose, and Sucrose from 25 to 40 °C | Journal of Chemical & Engineering Data

The importance of ion interactions on electrolyte solution viscosities determined by comparing concentrated sodium carbonate and nitrate solutions - ScienceDirect

Density, Viscosity, and Surface Tension of Sodium Carbonate + Sodium Bicarbonate Buffer Solutions in the Presence of Glycerine, Glucose, and Sucrose from 25 to 40 °C | Journal of Chemical & Engineering Data

Density and N2O solubility of sodium and potassium carbonate solutions in the temperature range 25 to 80 °C - ScienceDirect

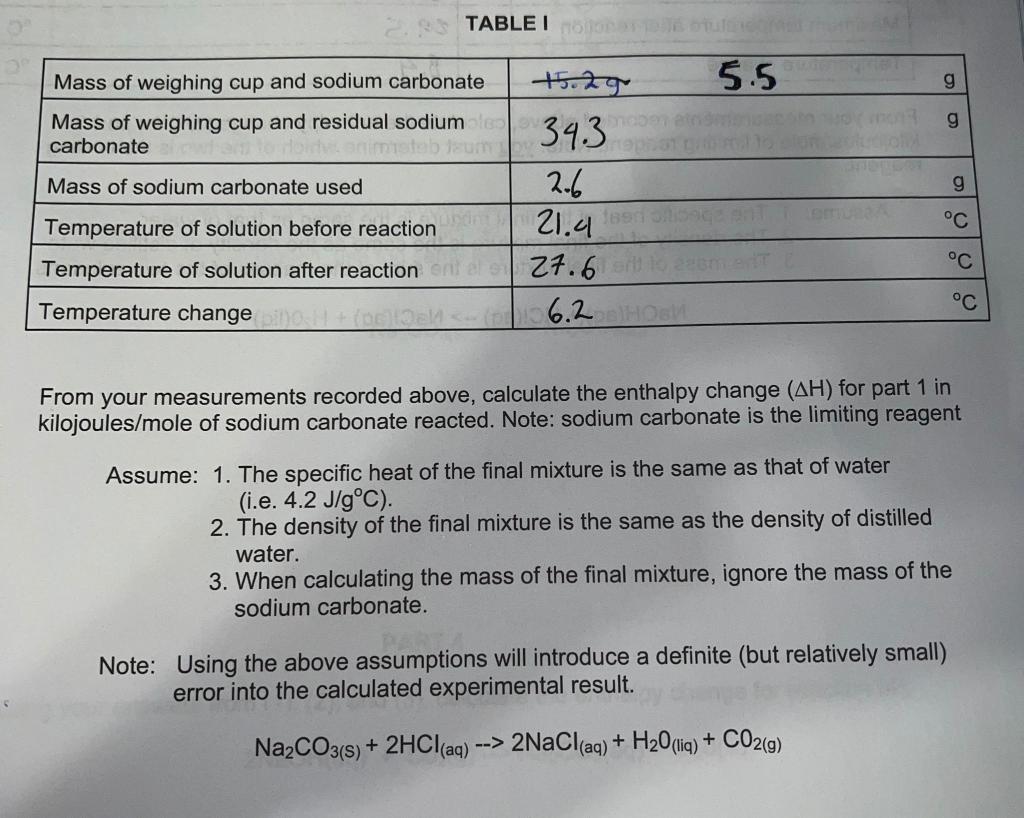
CCCCXXIII.—The heat of solution of sodium carbonate and the specific heats of its solutions - Journal of the Chemical Society (Resumed) (RSC Publishing)

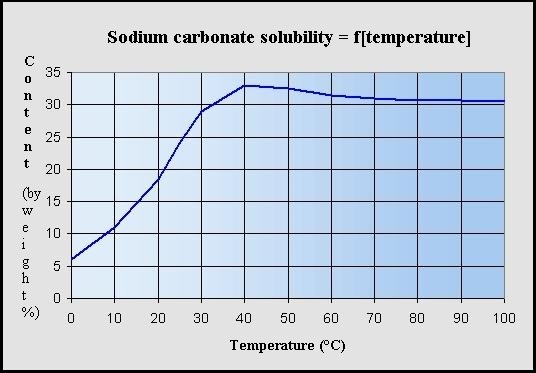
Effect of sodium carbonate concentration on Cl and F content in the... | Download Scientific Diagram
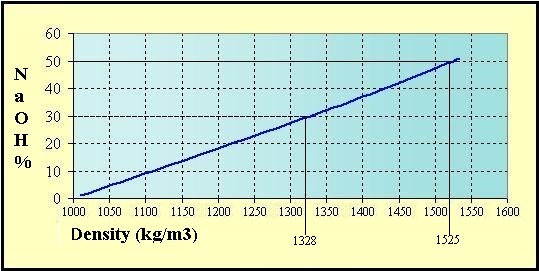
37. The strength of (10power 2)M Na2CO3 solution in term of molality will be (density of solution = 1.1 g/ml) (Molar mass of Na2CO3 = 106 g/mol)