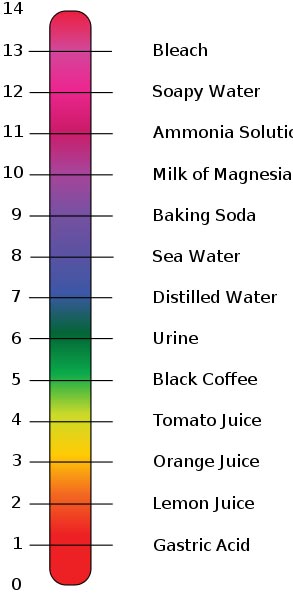
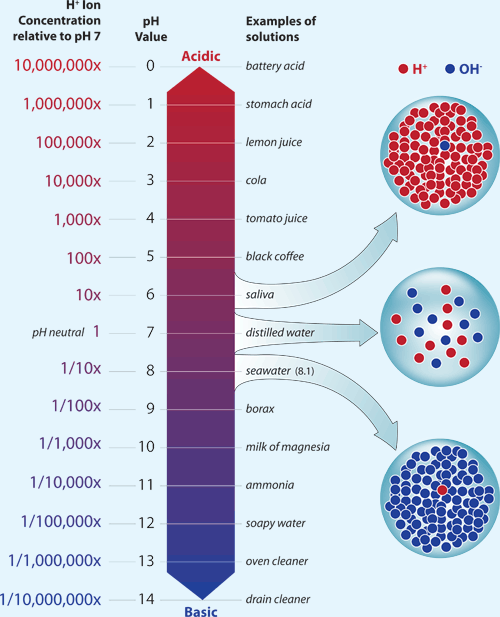
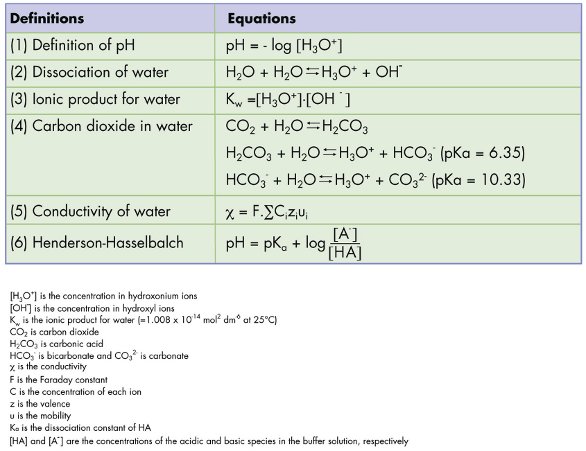
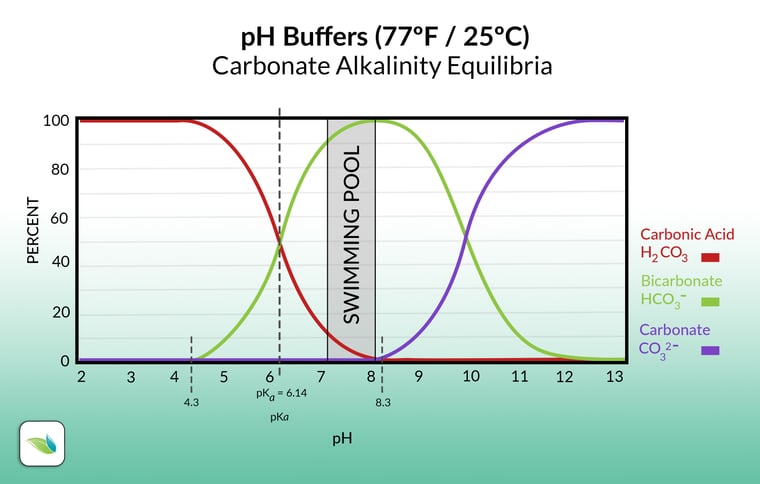
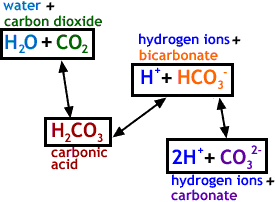
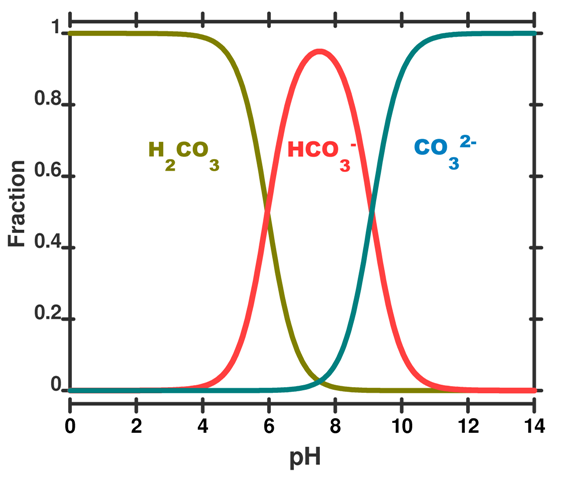
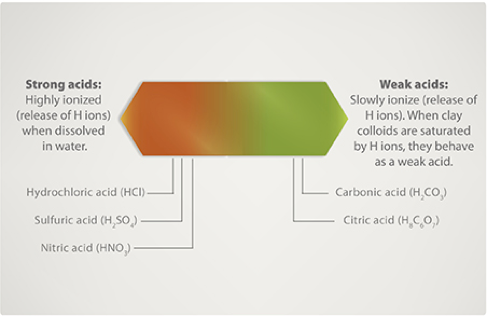
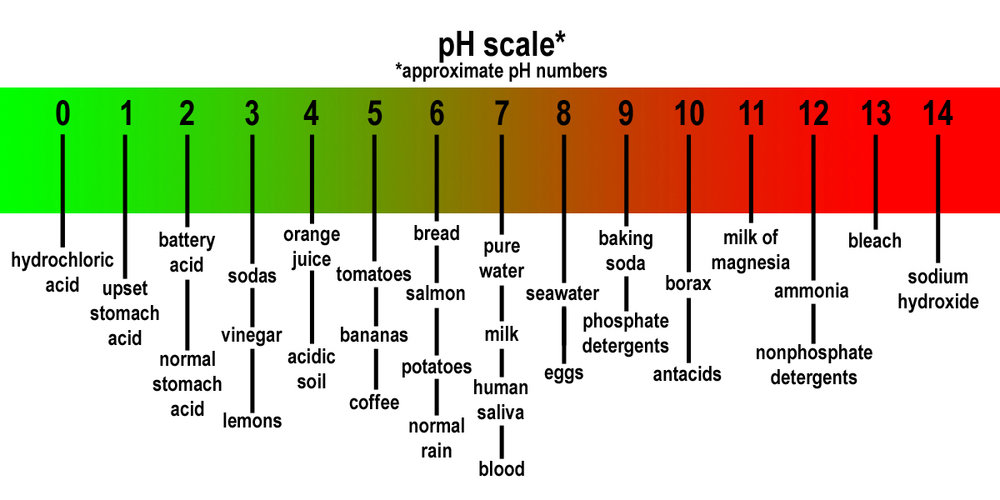
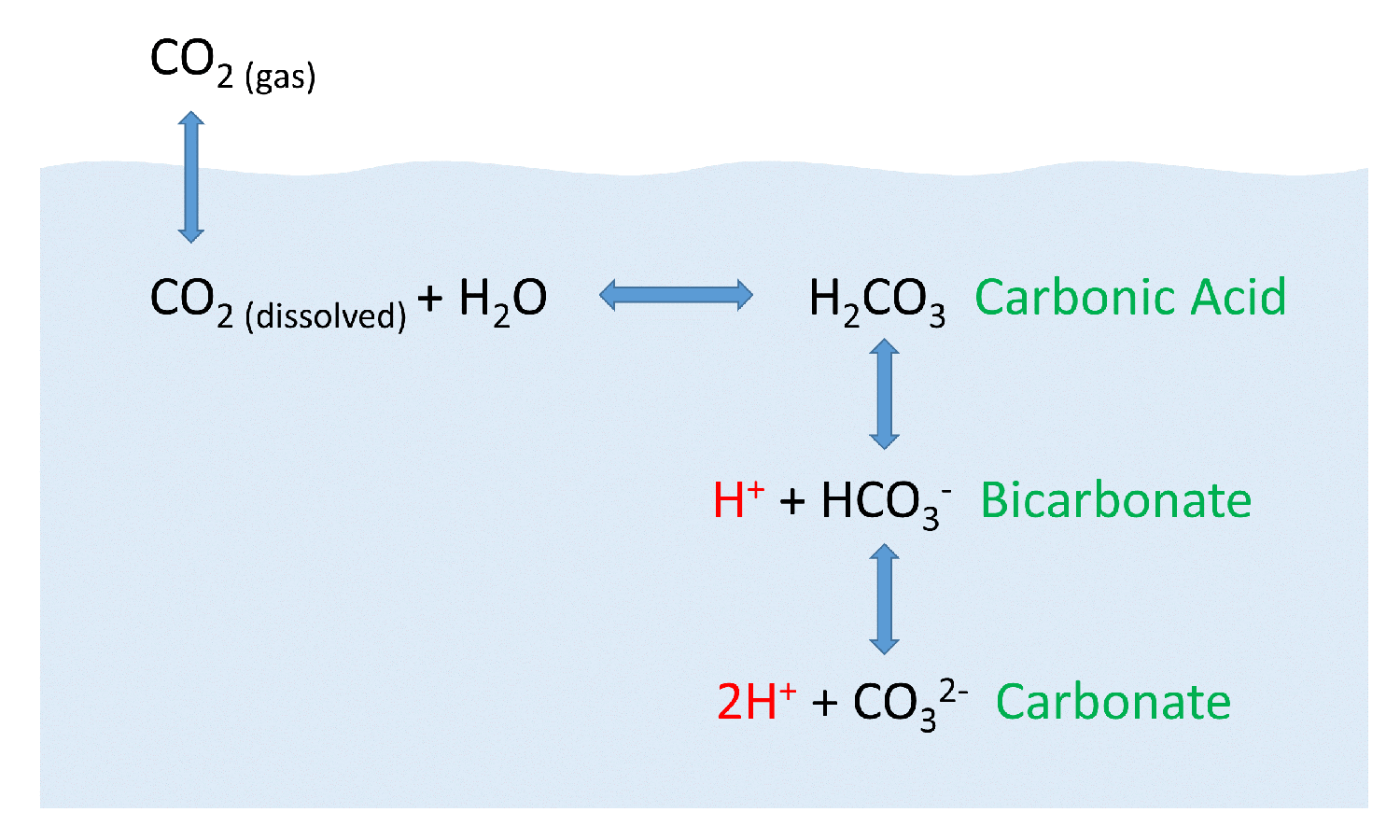
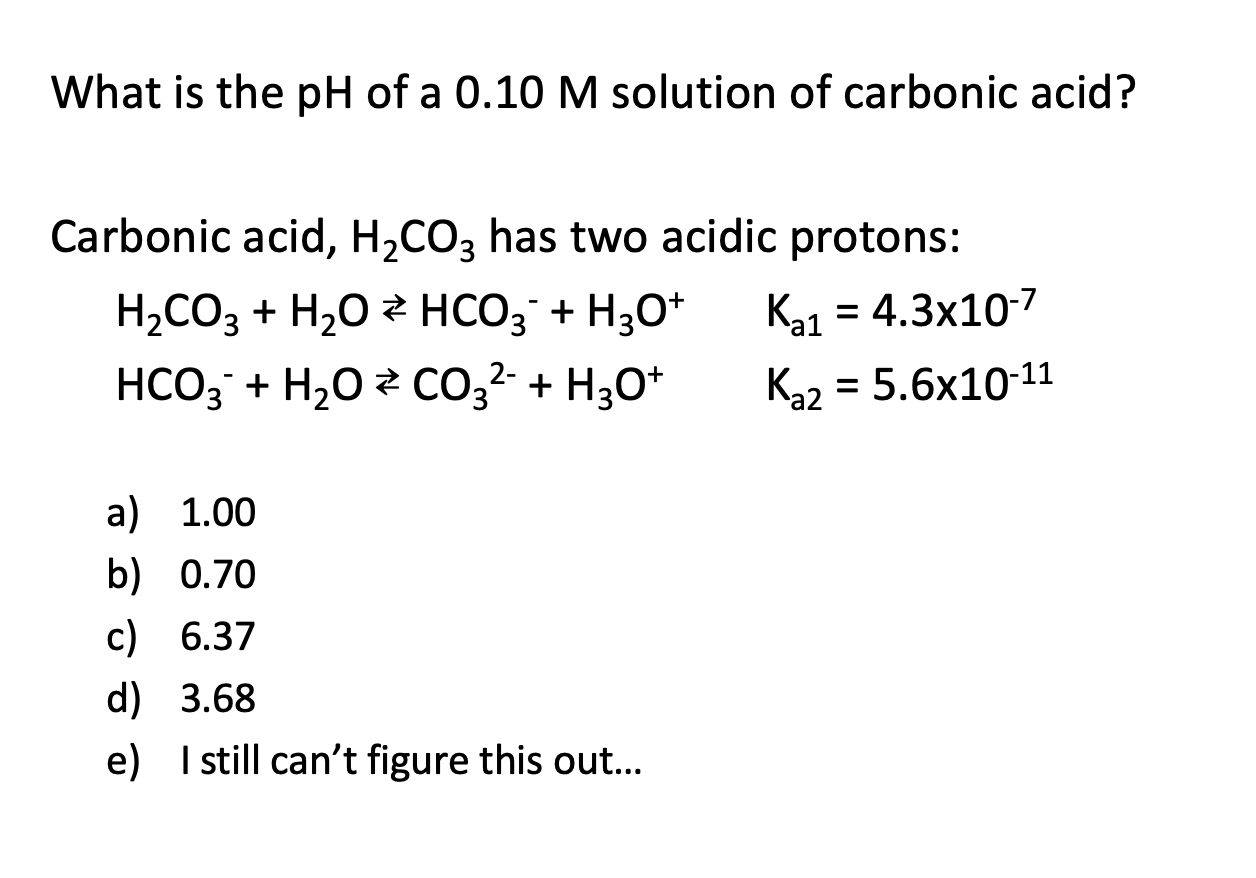
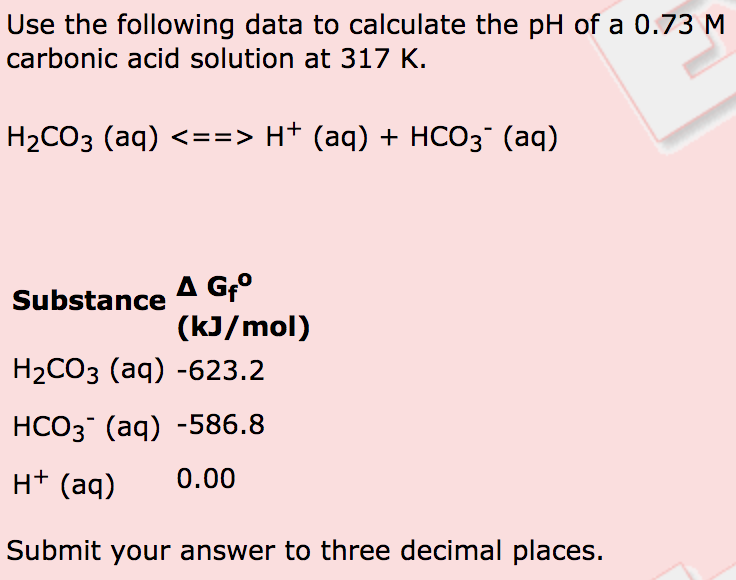
SOLVED: The pH of a bicarbonate-carbonic acid buffer is 6.62. Calculate the ratio of the concentration of carbonic acid ( H2CO3 ) to that of the bicarbonate ion ( HCO3− ). (
1 The speciation of carbonic acid (above) and phosphoric acid (under)... | Download Scientific Diagram