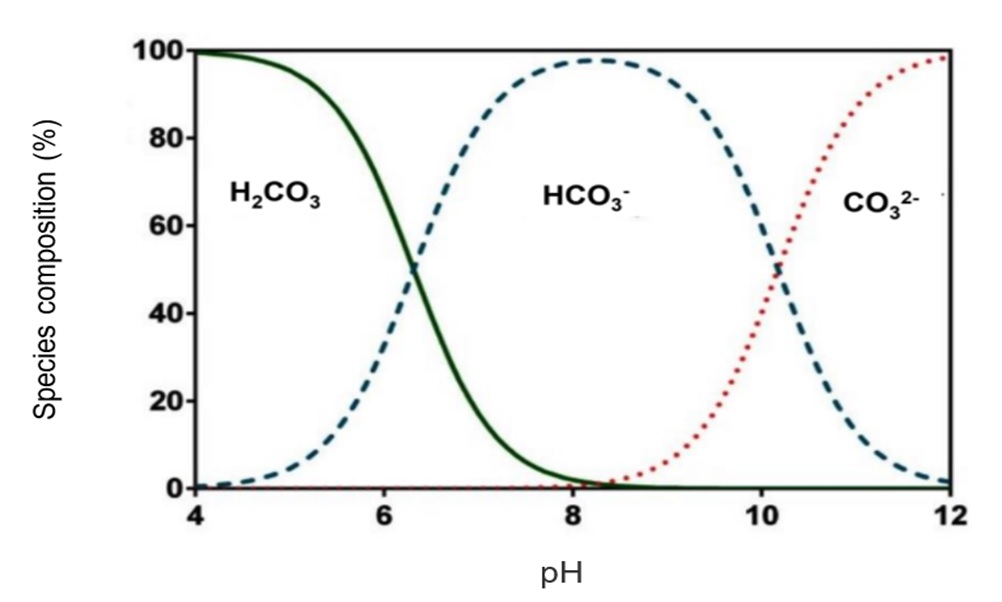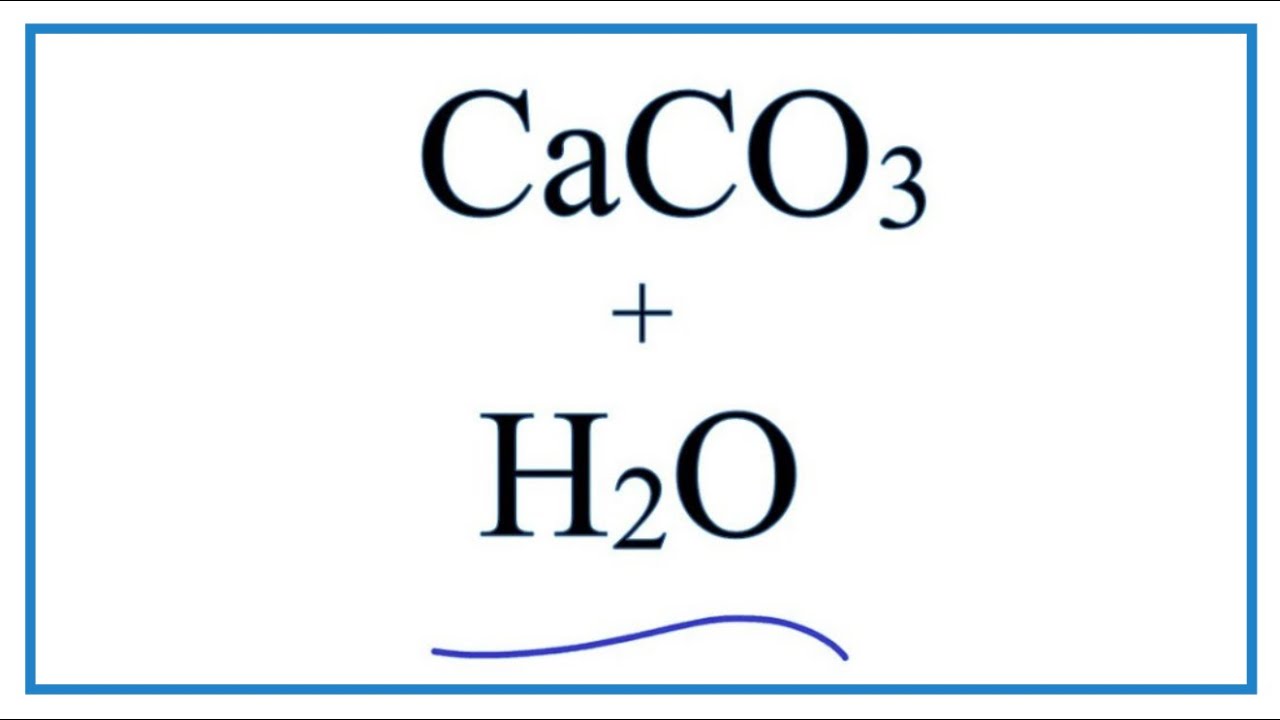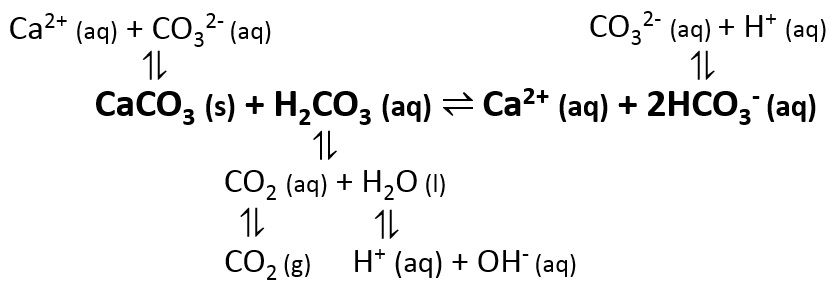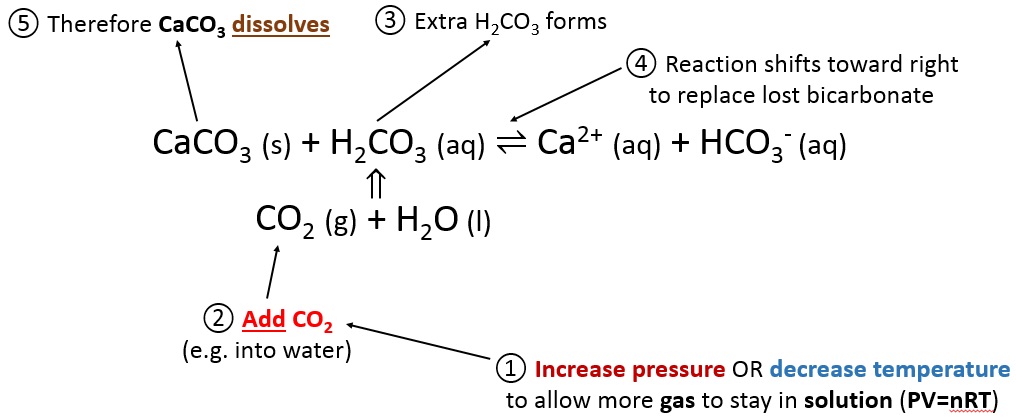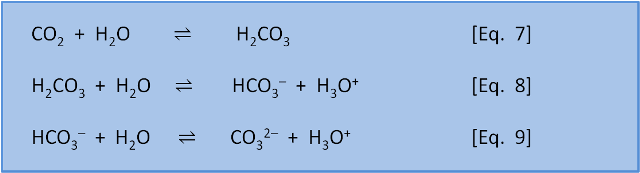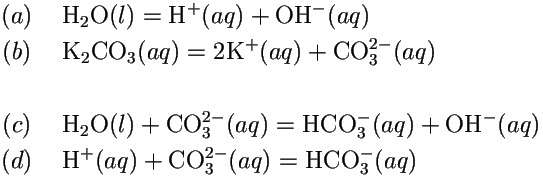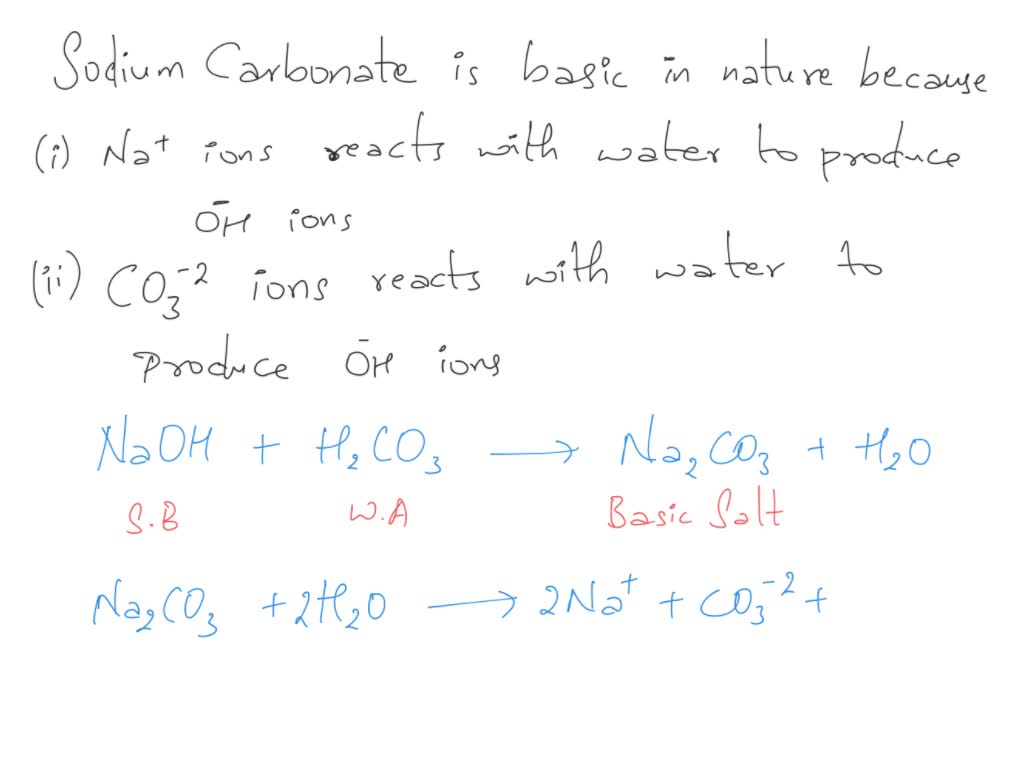
SOLVED: The best explanation for why sodium carbonate is basic is:sodium ions react with water to produce hydroxide ions ORCarbonate ions react with water to produce hydroxide ions
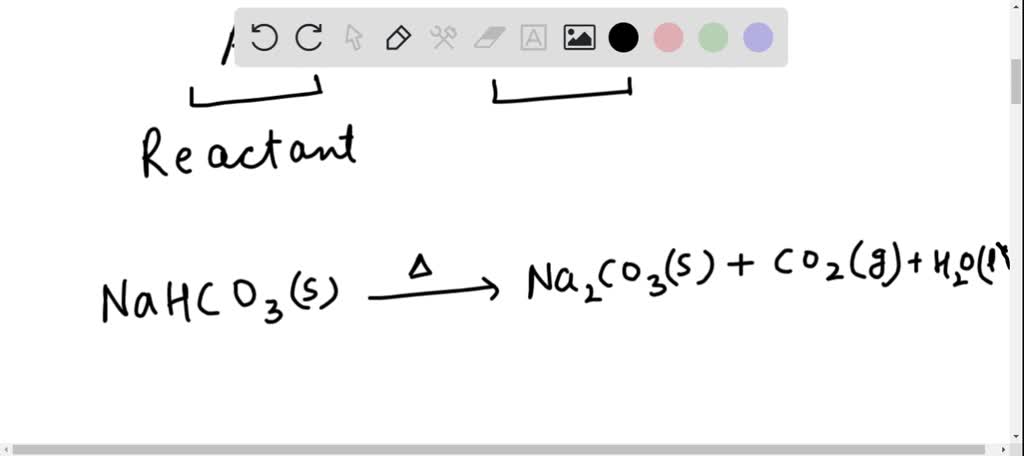
SOLVED: Write the balanced chemical reaction, including phase labels, for this reaction: "solid sodium bicarbonate decomposes into solid sodium carbonate, gaseous carbon dioxide, and liquid water."
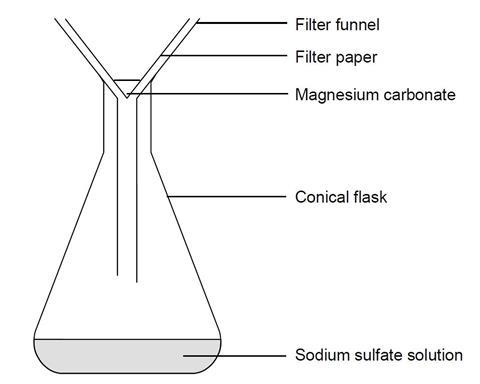
Making magnesium carbonate: the formation of an insoluble salt in water | Experiment | RSC Education

Summary of the reactions between carbon dioxide (CO2) with water (H2O)... | Download Scientific Diagram




![PDF] The Carbonate System in Swimming Pool Water | Semantic Scholar PDF] The Carbonate System in Swimming Pool Water | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/03bd630d0d7eaff721559678ce87e2f683c8eabf/4-Figure1-1.png)