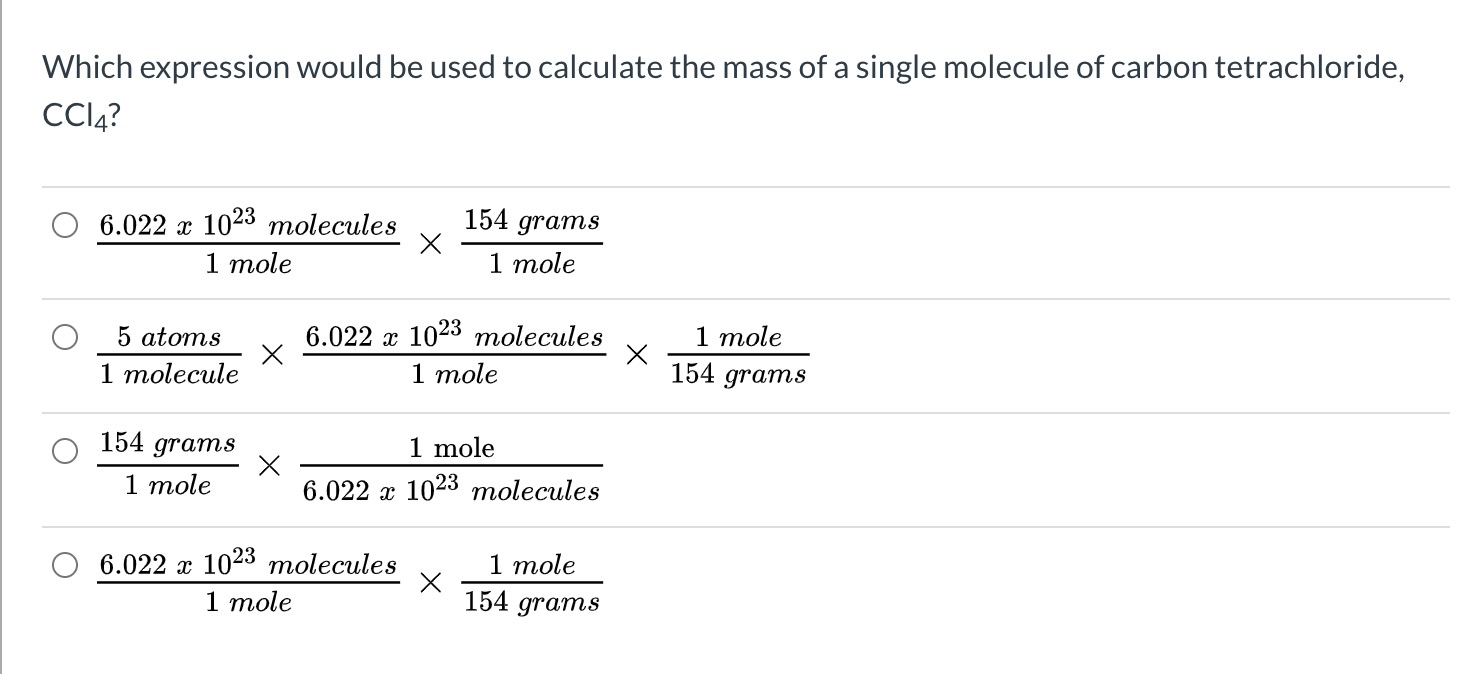Calculate the mole fraction of benzene in solution containing 30% by mass in carbon tetrachloride... - YouTube

Chapter 3 Chemical Quantities (The Mole). The Mole 1 dozen = 1 gross = 1 ream = 1 mole = x mole Carbon = g Carbon 6 C ppt download

Carbon reacts with chlorine to form CCl4· 36 gm of carbon was mixed with 142 gm of Cl2 . Calculate mass of CCl4 produced and the remaining mass of reactant.
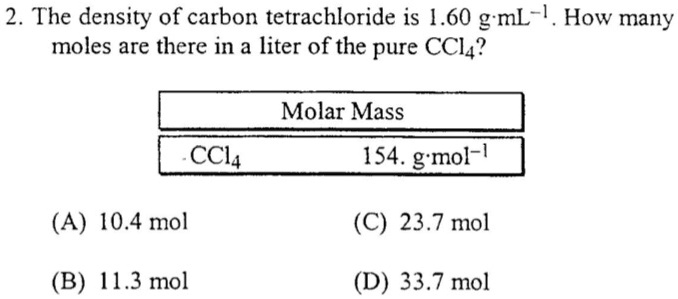
SOLVED: 2 The density of carbon tetrachloride is 1.60 g mL-1 How many moles are there in a liter of the pure CCl4? Molar Mass CCl4 154. g mol (A) 10.4 mol (

Carbon reacts with chlorine to form CCl4 . 36 gm of carbon was mixed with 142 g of Cl2 . Calculate the mass of CCl4 produced in the reaction.

If 0.48 g of sulfur are added to 200 g of carbon tetracholoride, and the freezing point of the carbon tetrachloride (K_f = 30 degrees C/m) is depressed by 0.28 degrees C,
The molar mass of CCl4 is approximately 153.8 g mol*-1. Is this the mass of only 1 molecule or 6.0 × 10*23 molecules of CCl4? - Quora

Chemical Quantities. Calculate the mass of compounds. Calculate the volume of a given mass of a gas from its density at a given temperature and pressure. - ppt download

The atomic masses of carbon and chlorine are12 01u and 35 46u respectively What is the molecular mass of - Chemistry - Structure of Atom - 1006910 | Meritnation.com

The Mole Unit 5. Formula Mass Formula mass - also called: formula massmolecular mass molecular massformula weight formula weightmolecular weight molecular. - ppt download

Calculate the mole fraction of benzene in solution containing 30% by mass in carbon tetrachloride. – The Unconditional Guru