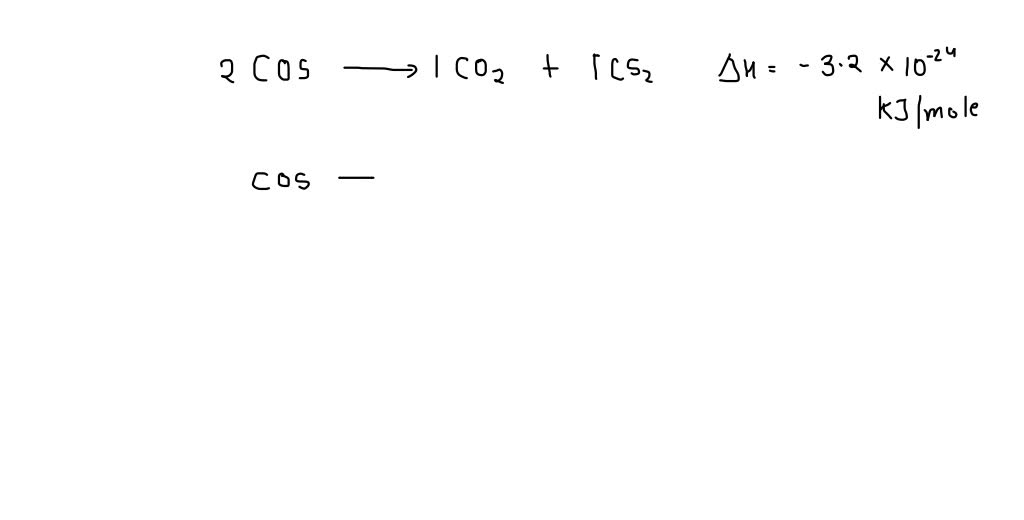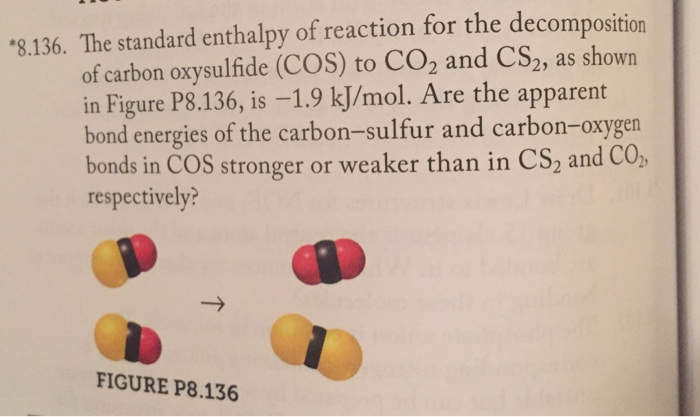SOLVED:The standard enthalpy of reaction for the decomposition of carbon oxysulfide (COS) to CO2 and CS2, as shown in Figure P 8.136, is -1.9 kJ / mol . Are the apparent bond
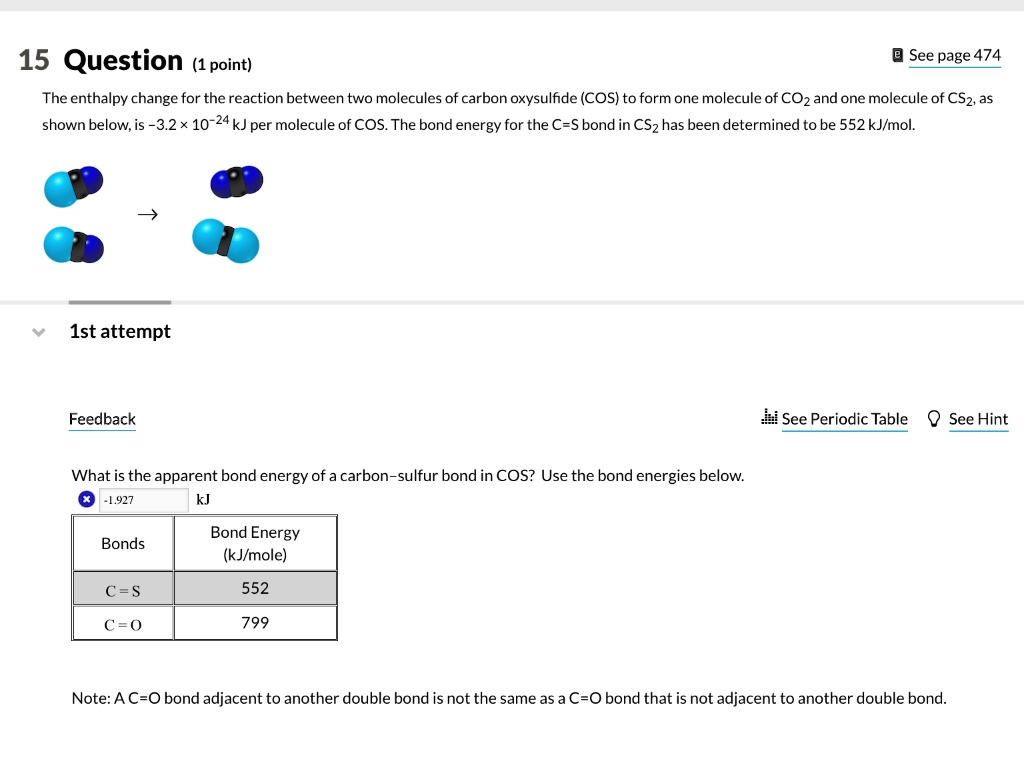
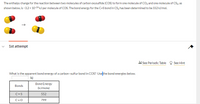
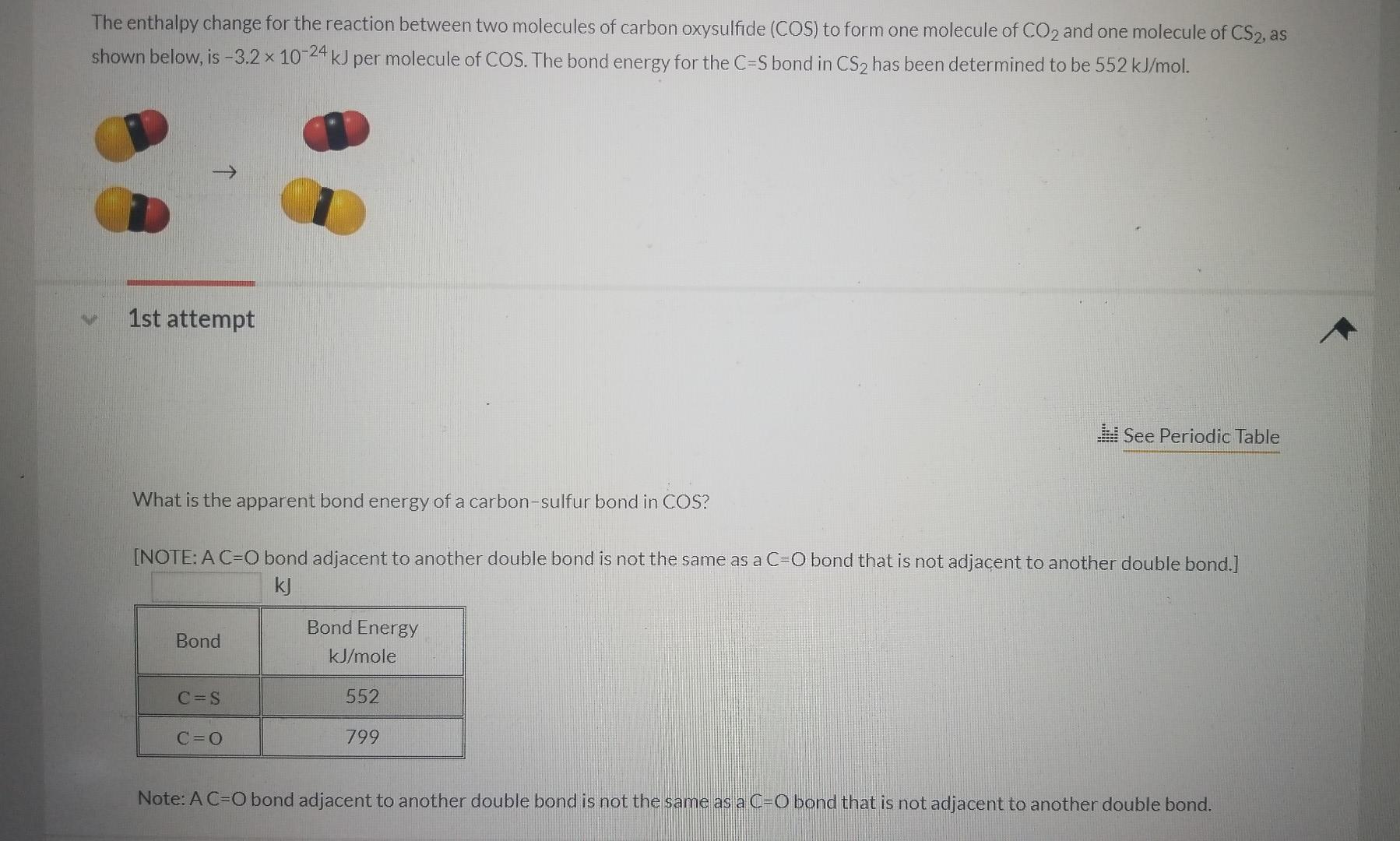
SOLVED: 15 Question (1 point) See page 474 The enthalpy change for the reaction between two molecules of carbon oxysulfide (COS) to form one molecule of CO2 and one molecule of CS2,
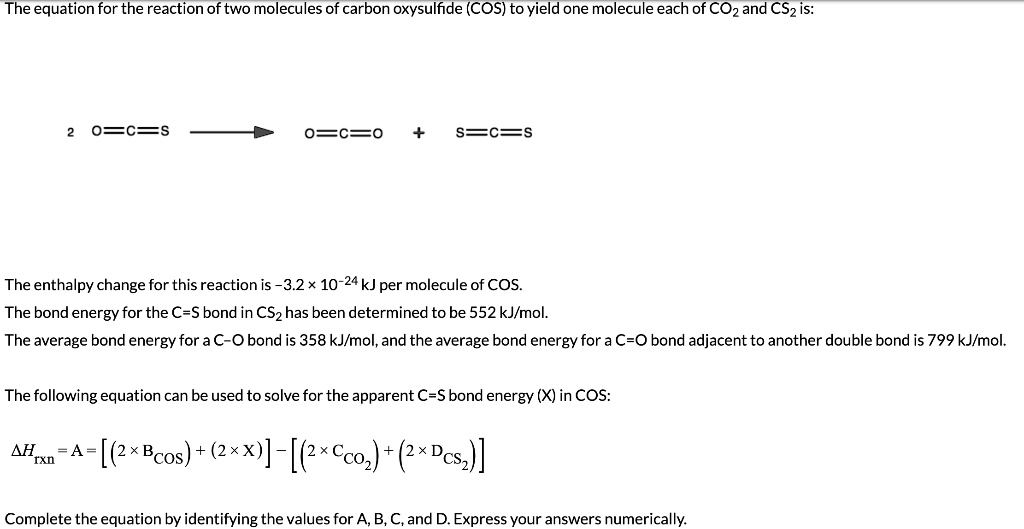
SOLVED: The equation for the reaction of two molecules of carbon oxysulfide (COS) to yield one molecule each of COz = and CSzis: O=C=O S=C=5 The enthalpy change for this reaction is

Emerging Roles of Carbonyl Sulfide in Chemical Biology: Sulfide Transporter or Gasotransmitter? | Antioxidants & Redox Signaling

Periodic table showing the reported oxysulfide compounds. In blue are... | Download Scientific Diagram
Reference Exposure Levels (RELs) for carbonyl sulfide (COS) for use in the Air Toxics Hot Spots Program
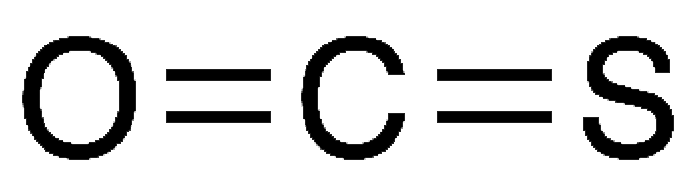
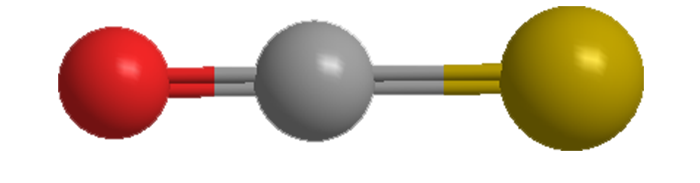
SOLVED:The connectivity of carbon oxysulfide is OCS. (a) Write a Lewis formula for carbon oxysulfide that satisfies the octet rule. (b) What is the molecular geometry according to VSEPR? (c) Does carbon

Photochemical production of carbonyl sulfide, carbon disulfide and dimethyl sulfide in a lake water - ScienceDirect