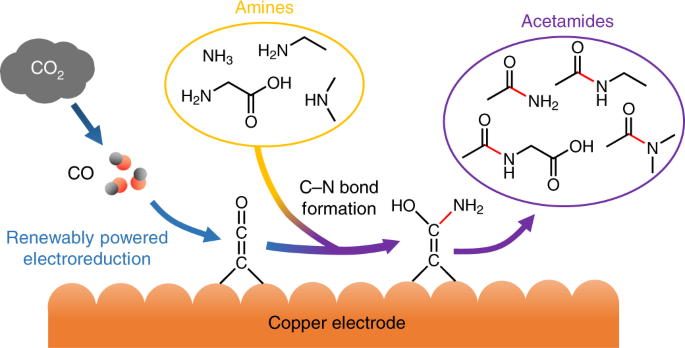
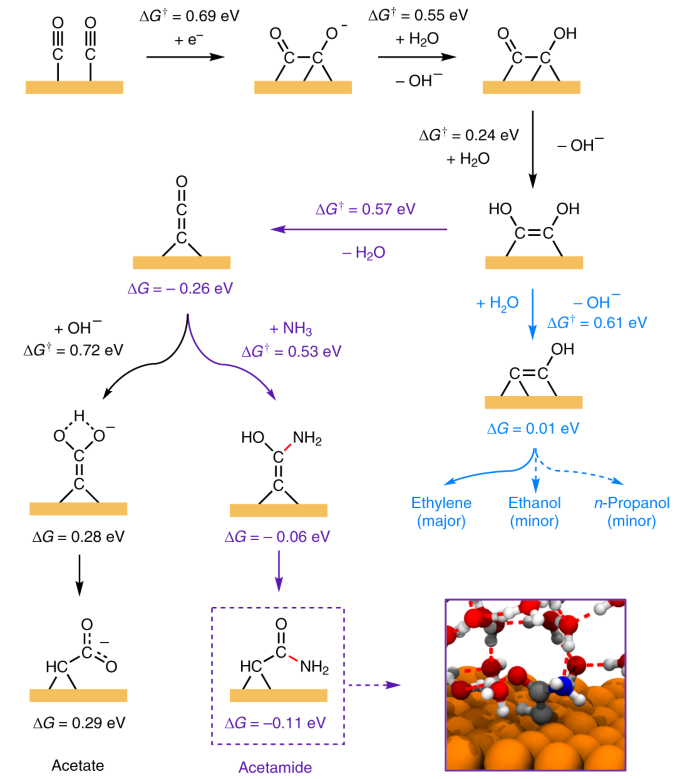
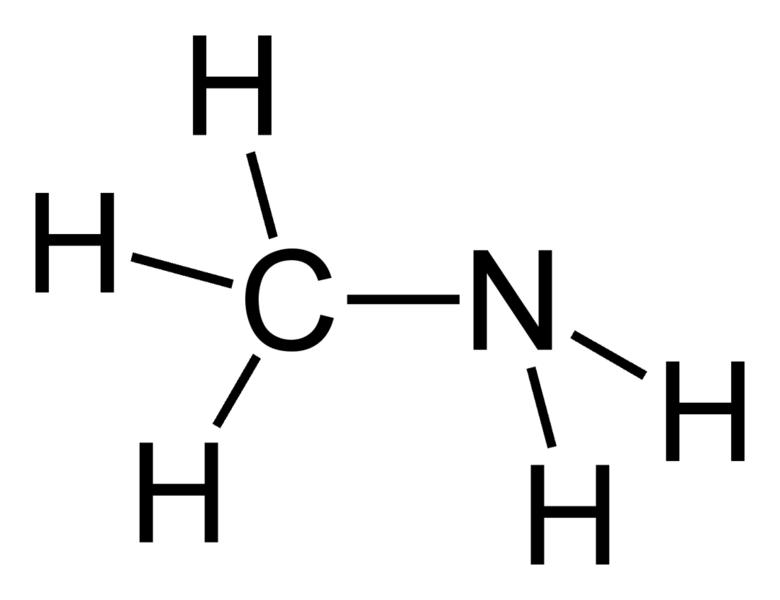
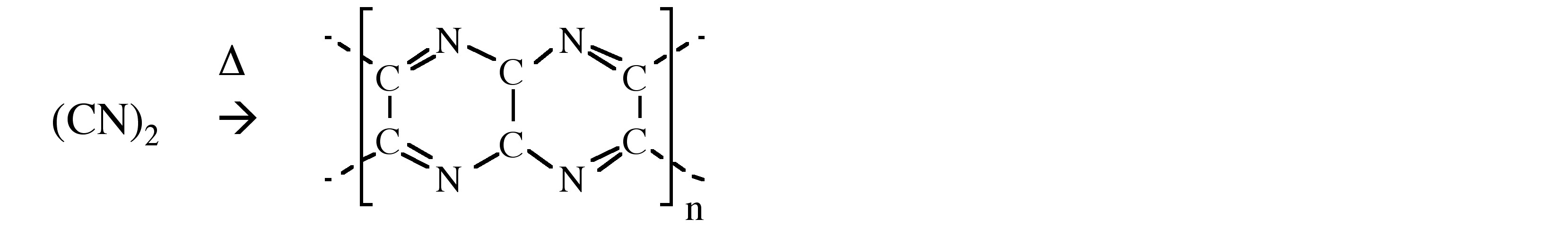A compound of carbon, hydrogen and nitrogen contains these elements in the ratio 9:1:3.5. Calculate the empirical formula. - Sarthaks eConnect | Largest Online Education Community

Following the rule that each atom of carbon, oxygen, and nitrogen reacts to achieve a complete outer shell of eight valence electrons, add unshared pairs of electrons as necessary to complete the
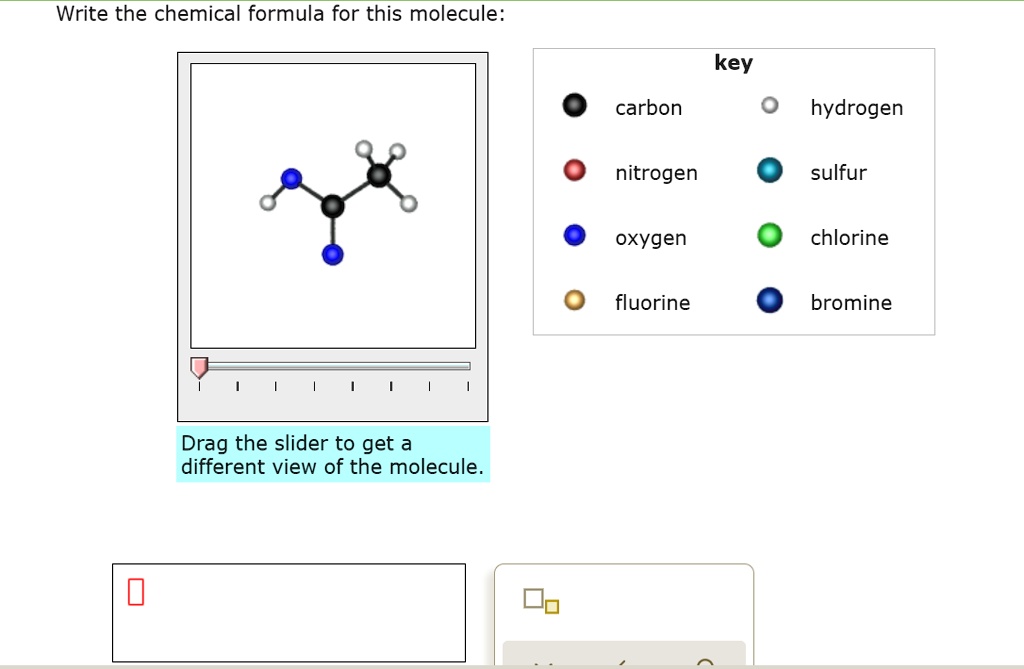
SOLVED: Write the chemical formula for this molecule: carbon hydrogen nitrogen sulfur oxygen chlorine fluorine bromine Drag the slider to get a different view of the molecule key

Inorganic Compounds of Carbon, Nitrogen, and Oxygen - Leikauf - Major Reference Works - Wiley Online Library

What is the empirical formula for a compound containing 38.8% carbon, 16.2% hydrogen and 45.1% nitrogen?

An organic compound contains 58.55% carbon, 4.05% hydrogen and 11.36% nitrogen. If its vapour density is 61.5 . The molecular formula is C6H5NO2 .

Chemical formulas and space structures of selected compounds. Carbon... | Download Scientific Diagram

Propanil herbicide molecule. Stylized skeletal formula (chemical structure): Atoms are shown as color-coded circles: hydrogen (hidden), carbon (grey), nitrogen (blue), oxygen (red), chlorine (green Stock Photo - Alamy

An organic compound contains 20 % carbon, 6.7 % hydrogen, and 46.67 % nitrogen. Its molecular weight was found to be 60 . Find the molecular formula of the compound.