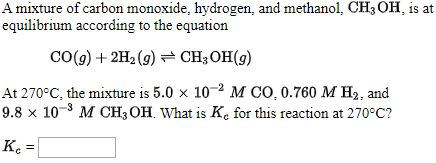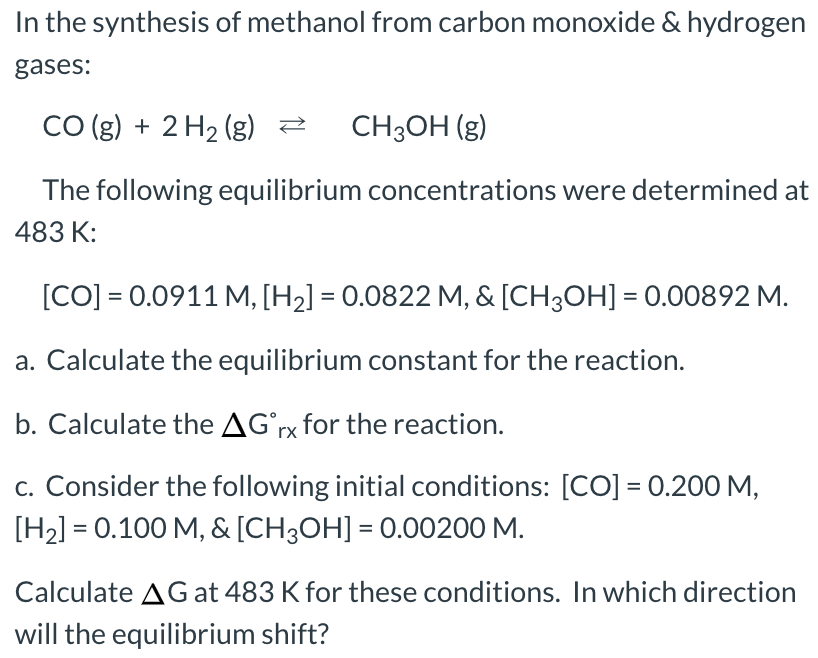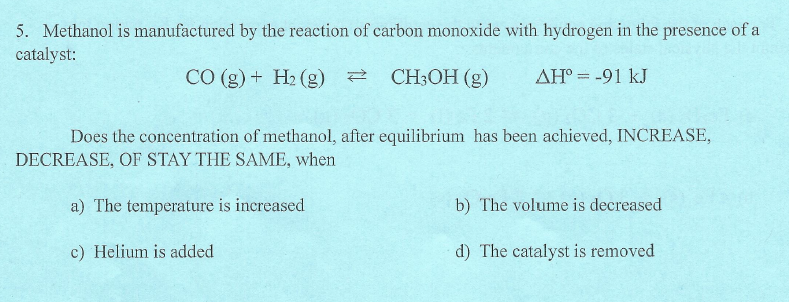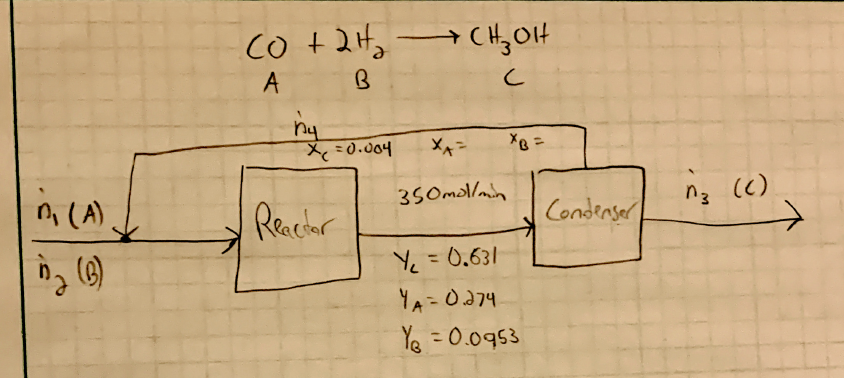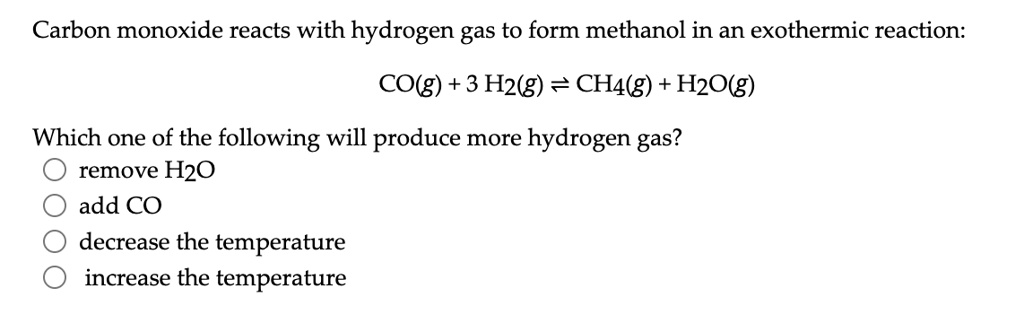
SOLVED: Carbon monoxide reacts with hydrogen gas to form methanol in an exothermic reaction: CO(g) 3 Hz(g) = CH4(g) + H2Okg) Which one of the following will produce more hydrogen gas? remove

EP0113709B1 - Method in the production of methyl formate and methanol in a liquid phase - Google Patents

Alcohol-Assisted Hydrogenation of Carbon Monoxide to Methanol Using Molecular Manganese Catalysts | JACS Au

Carbon monoxide reacts with hydrogen under certain conditions to from methanol `(CH_(3)OH)` - YouTube

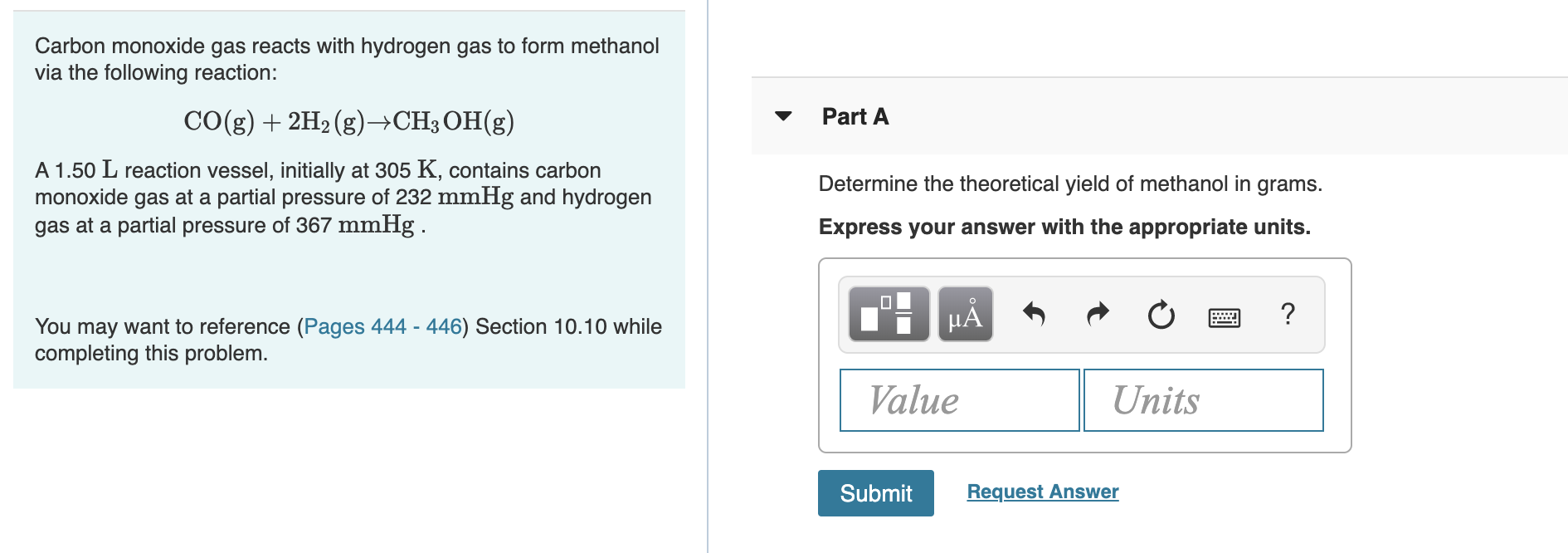
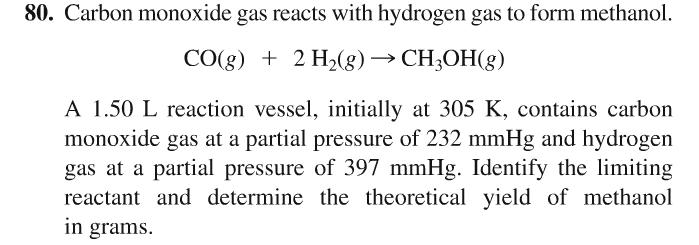
Methanol can be prepared synthetically by heating carbon monoxide and hydrogen gases under pressure in the presence of a catalyst. The reaction is CO(g) + 2H2(g)→CH3)H(l) Determine the enthalpy of this reaction
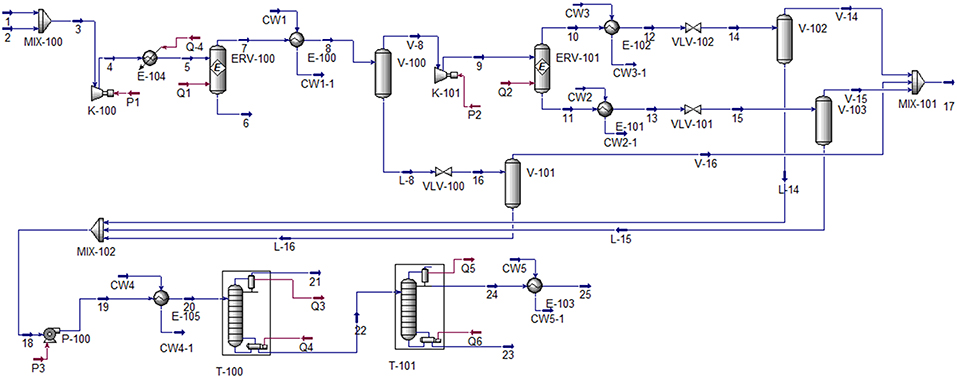
Frontiers | Methanol Production via CO2 Hydrogenation: Sensitivity Analysis and Simulation—Based Optimization

hydrocarbons - Why carbon monoxide reacts with hydrogen to give different products in the presence of different catalysts - Chemistry Stack Exchange

Methanol production via direct carbon dioxide hydrogenation using hydrogen from photocatalytic water splitting: Process development and techno-economic analysis - ScienceDirect
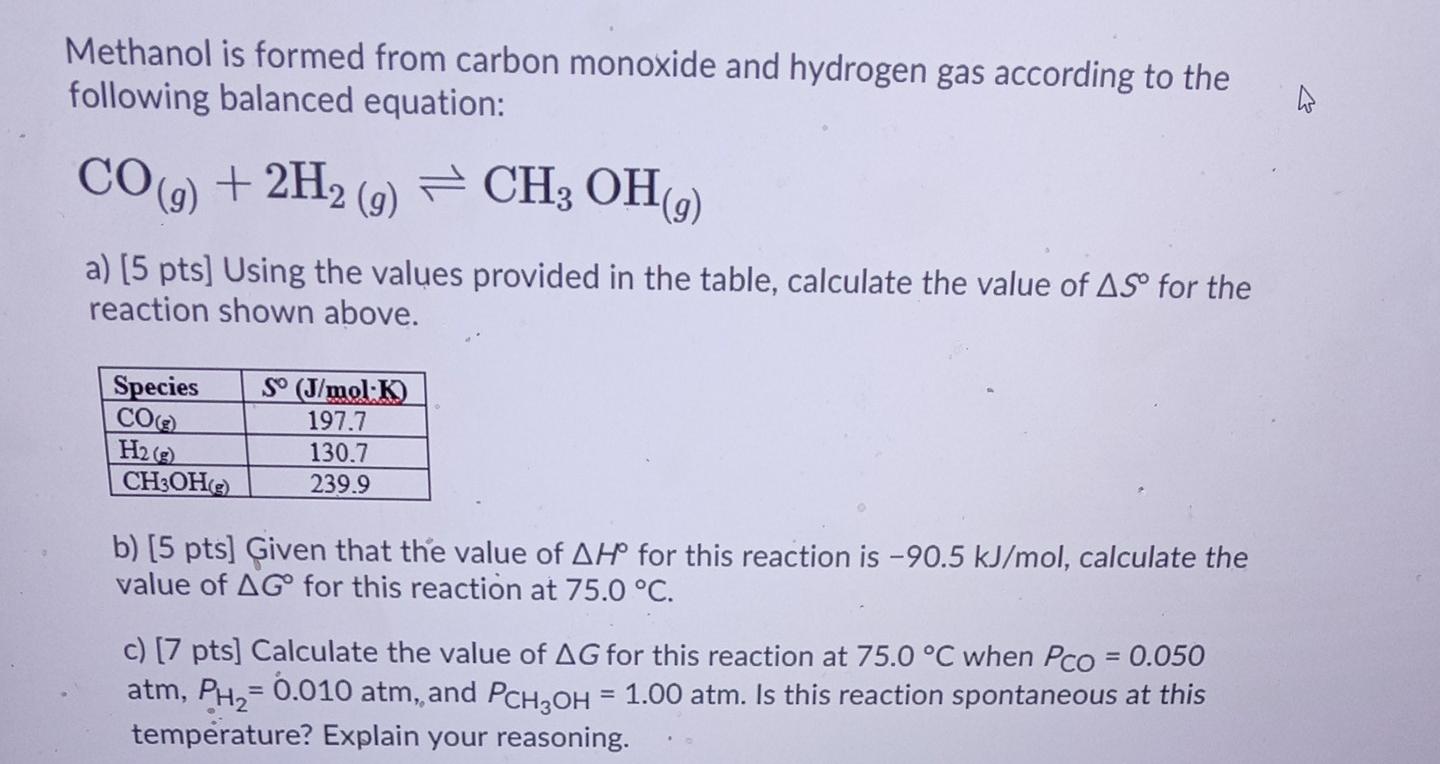
![SOLVED: Methanol is formed from carbon monoxide and hydrogen gas according to the following balanced equation: CO(g) 2Hz (9) CHs ' OH(g) a) [5 pts] Using the values provided in the table; SOLVED: Methanol is formed from carbon monoxide and hydrogen gas according to the following balanced equation: CO(g) 2Hz (9) CHs ' OH(g) a) [5 pts] Using the values provided in the table;](https://cdn.numerade.com/ask_images/16c04d240456462d8b6ebcc0b2d26908.jpg)
SOLVED: Methanol is formed from carbon monoxide and hydrogen gas according to the following balanced equation: CO(g) 2Hz (9) CHs ' OH(g) a) [5 pts] Using the values provided in the table;

carbon monoxide reacts with hydrogen under certain conditions to form Methanol write a balanced chemical - Brainly.in

![ANSWERED] The synthesis of methanol from carbon mon... - Physical Chemistry ANSWERED] The synthesis of methanol from carbon mon... - Physical Chemistry](https://media.kunduz.com/media/sug-question/raw/59241658-1659706634.3705795.jpeg)