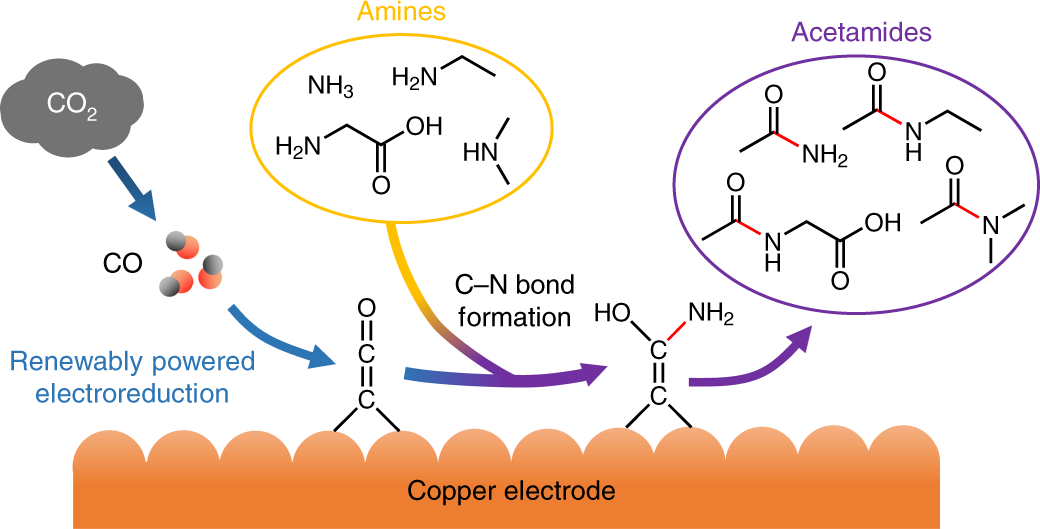
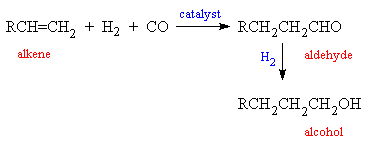Transition metal–free ketene formation from carbon monoxide through isolable ketenyl anions | Science

41524992Given standard enthalpy of formation of `CO(-110 \"KJ mol\"^(-1))` and `CO_(2)(-394 \"KJ mol - YouTube

The energy levels of carbon monoxide molecules and the formation of... | Download Scientific Diagram
66 Enthalpies of formation of co(g),CO2(g),N2O(g)and N2O4(g) are 110, 398, 81, 97 KJ/lol respectively. Find value of H for the reaction. N2O4(g) +3CO(g) — >N20(g)+3CO2(g)
the enthalpies of combustion of carbon and carbon monooxide are 393.5kJ and 283kJ ,respectively the enthalpy of formation of carbon monoxide 1. 676.5kJ 2. 110.5kJ 3.110.5kJ 4.676.5

Reductive Cleavage of the CO Molecule by a Reactive Vicinal Frustrated PH/BH Lewis Pair | Journal of the American Chemical Society

Phosgene formation via carbon monoxide and dichlorine reaction over an activated carbon catalyst: Towards a reaction model - ScienceDirect
62.The Enthalpies of combustion of carbon and carbon monoxide are 390 kJ and 278kJ respectively. The enthalpy of formation of carbon monooxide is? a) 669 kJ b) 112 kJ c) 112 kJ d) 668 kJ
Carbon Formation from Carbon Monoxide-Hydrogen Mixtures over Iron Catalysts.I. Properties of Carbon Formed | The Journal of Physical Chemistry