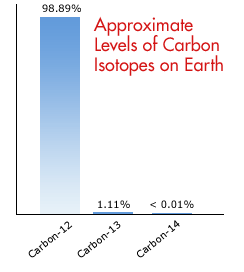
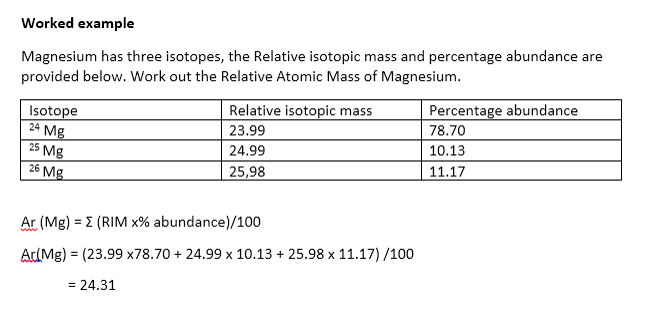
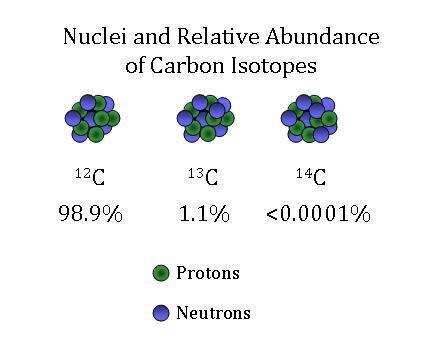
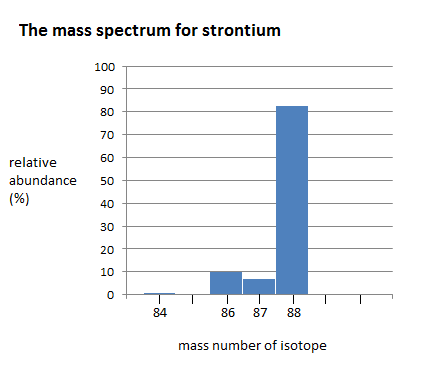
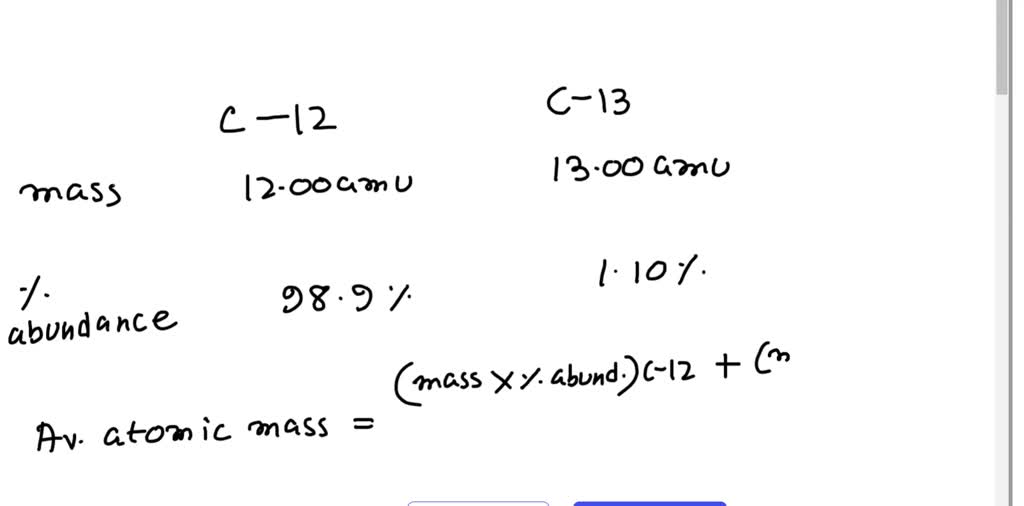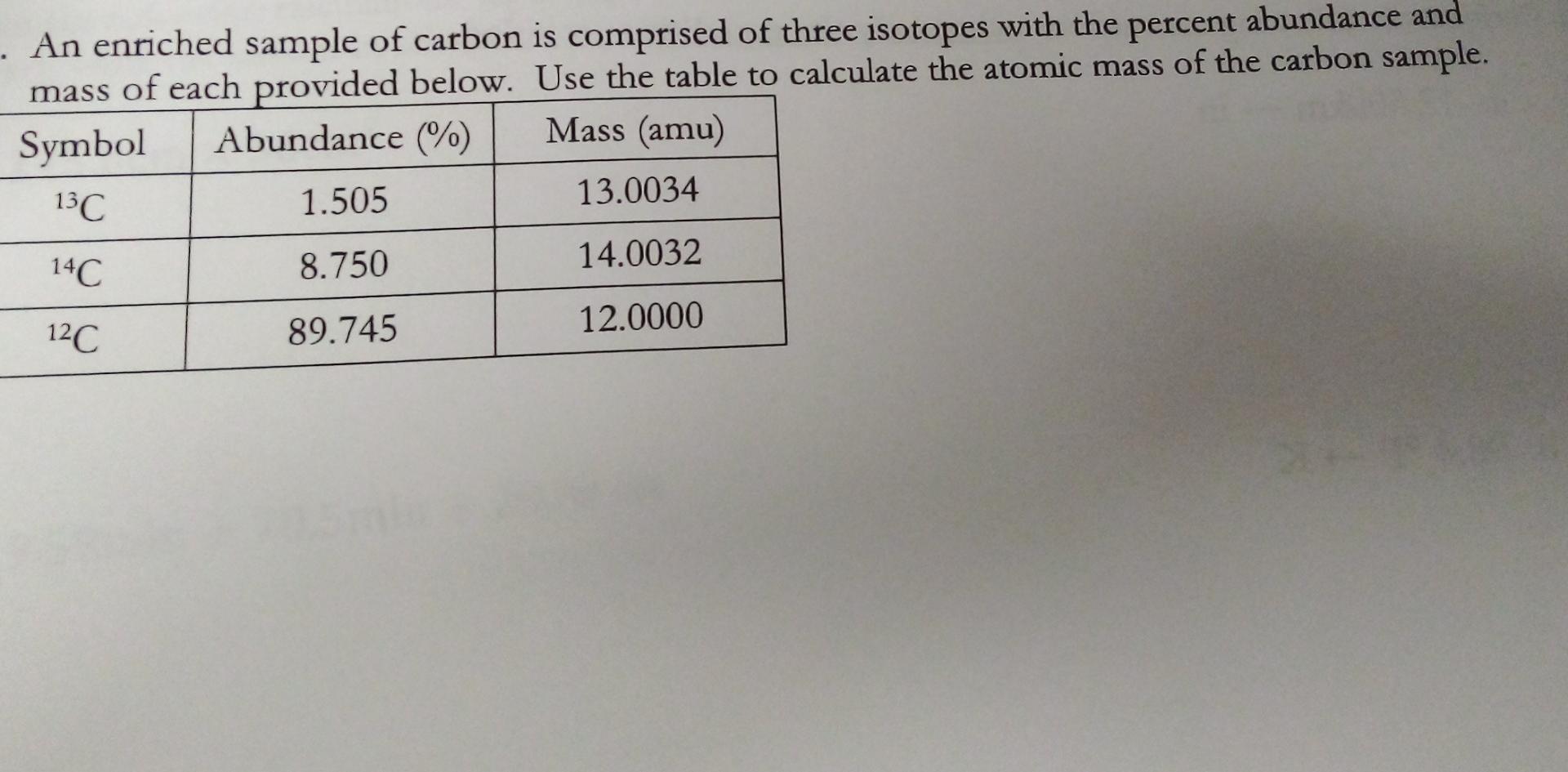The relative abundances of the stable isotopes of elements commonly... | Download Scientific Diagram

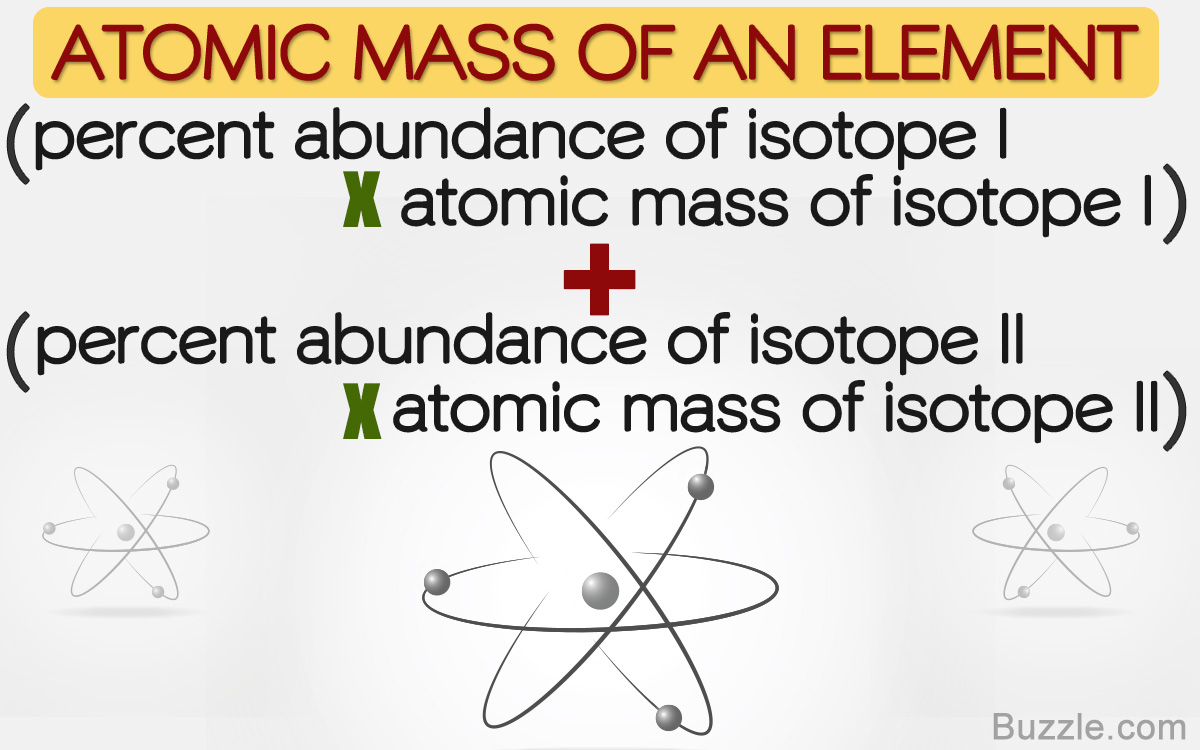
Isotopic Abundance SCH 3U. Atomic Mass The mass of an atom (protons, neutrons, electrons) Relative Atomic Mass: An element's atomic mass relative to the. - ppt download