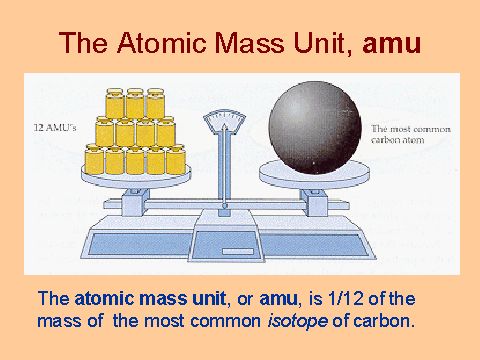
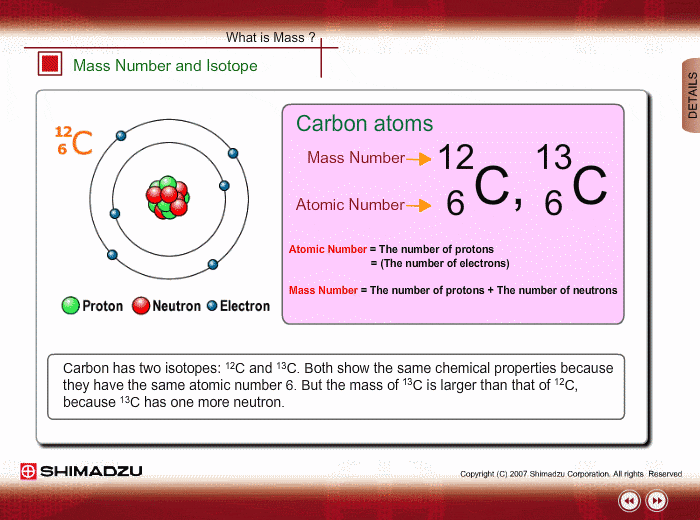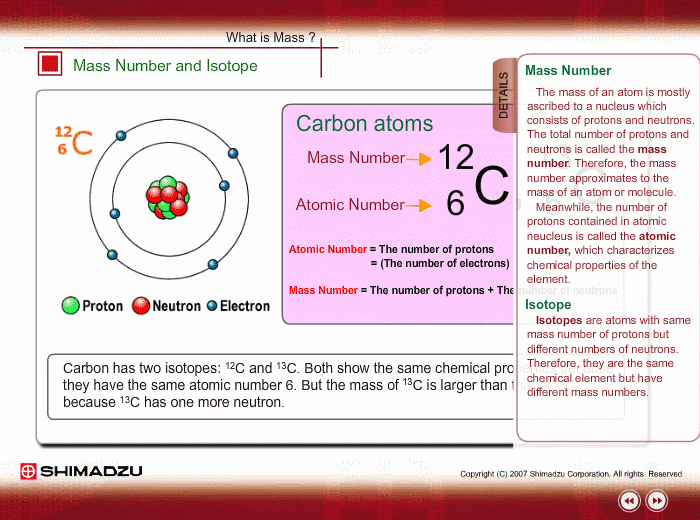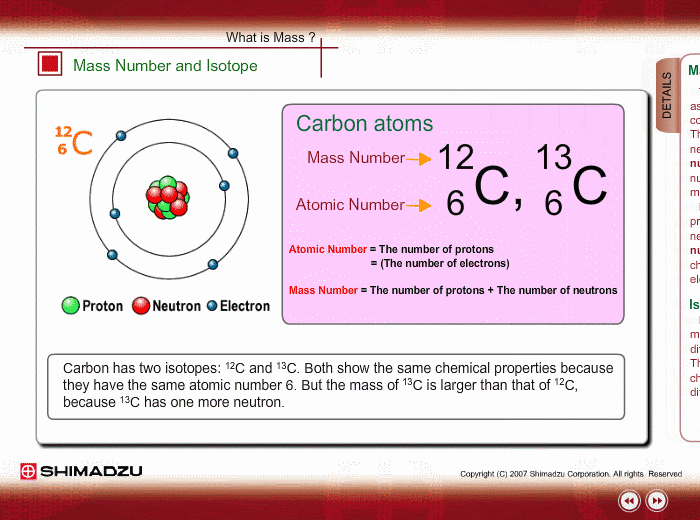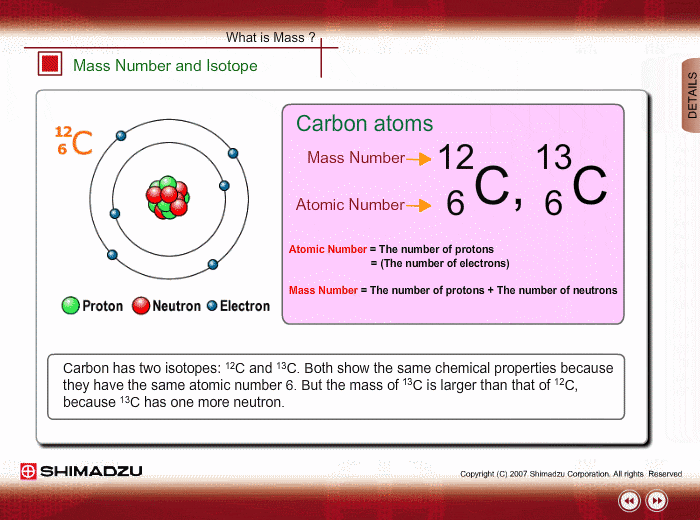
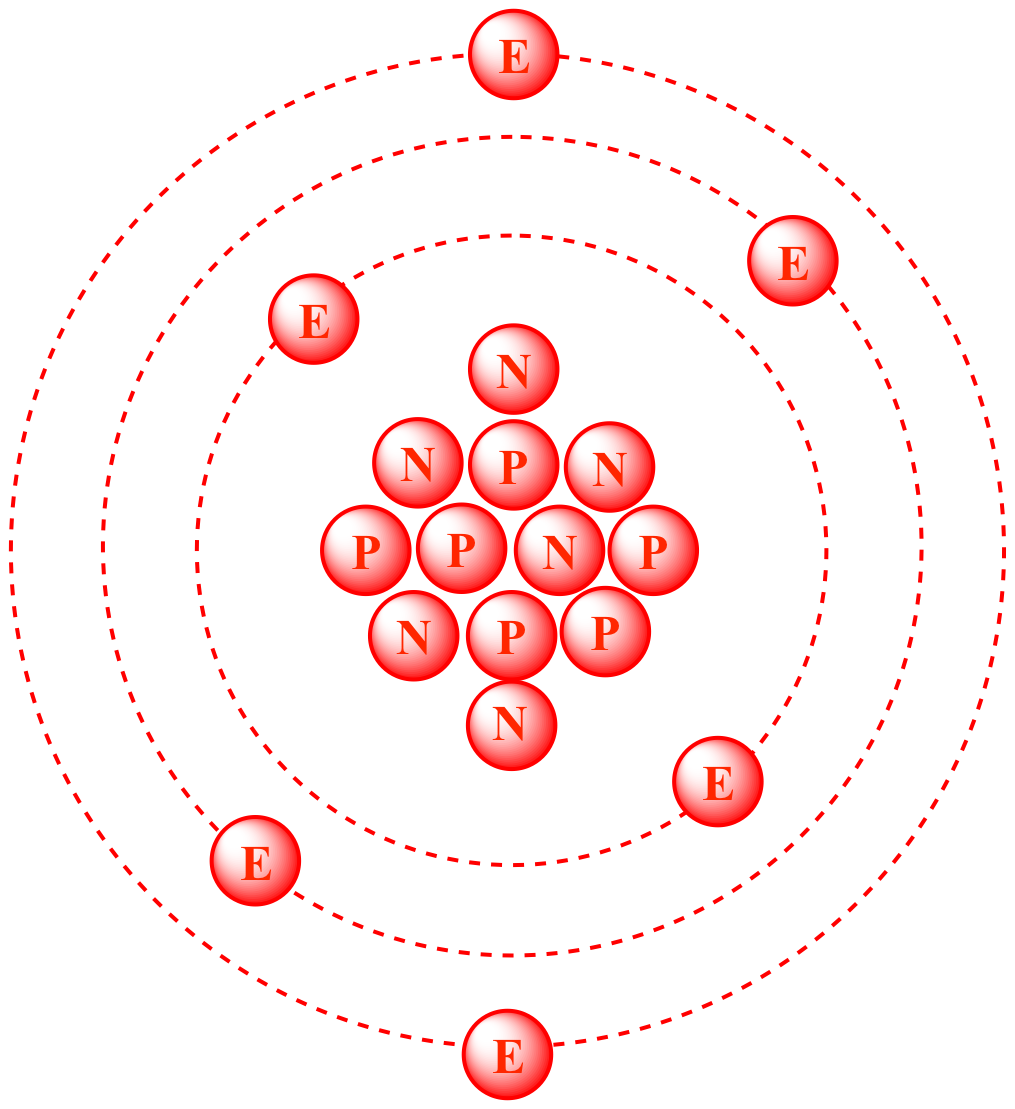
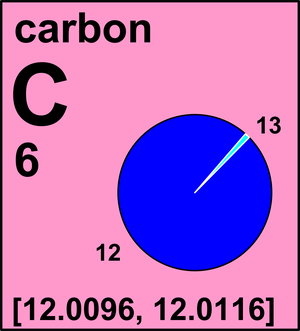
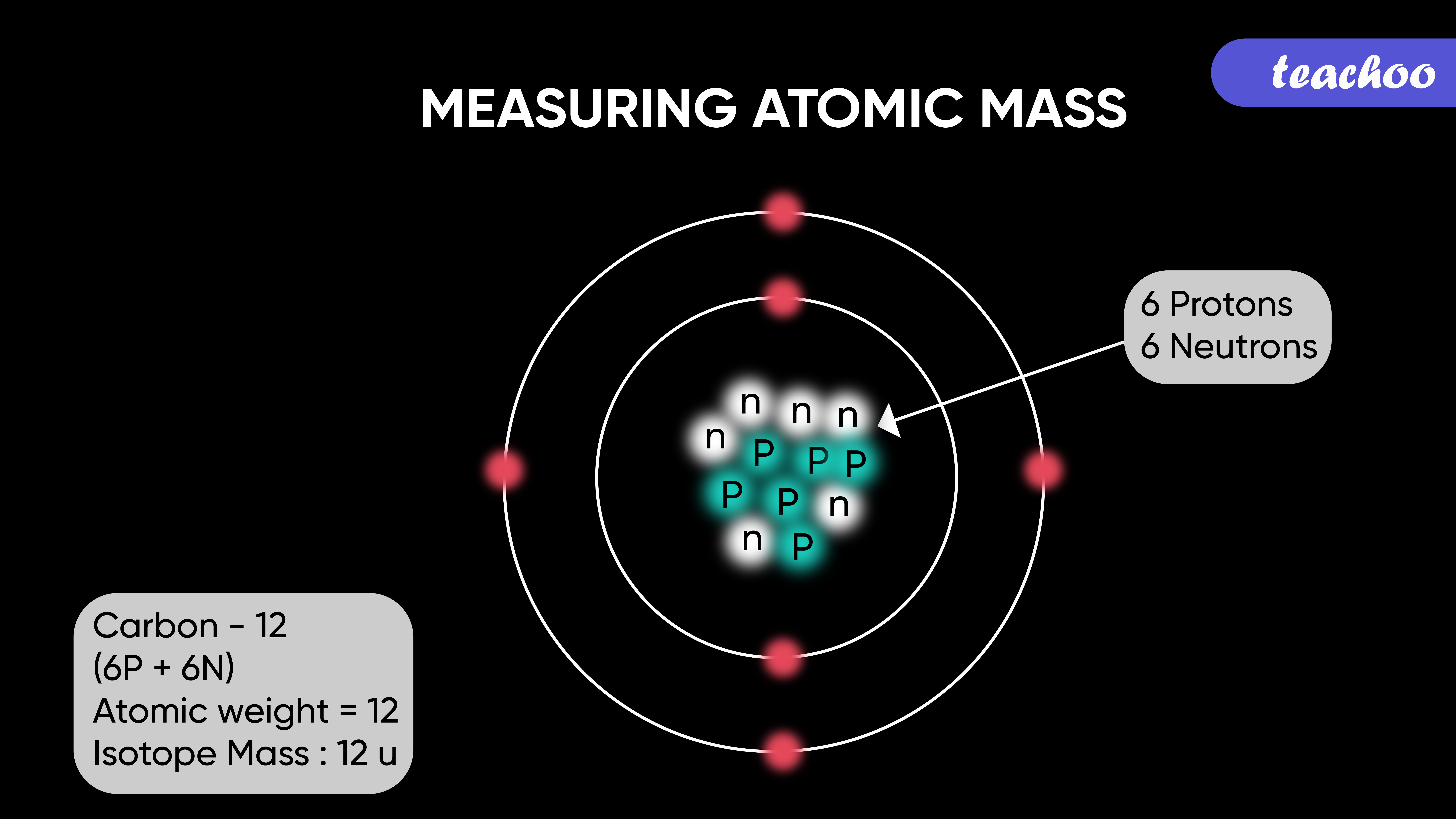
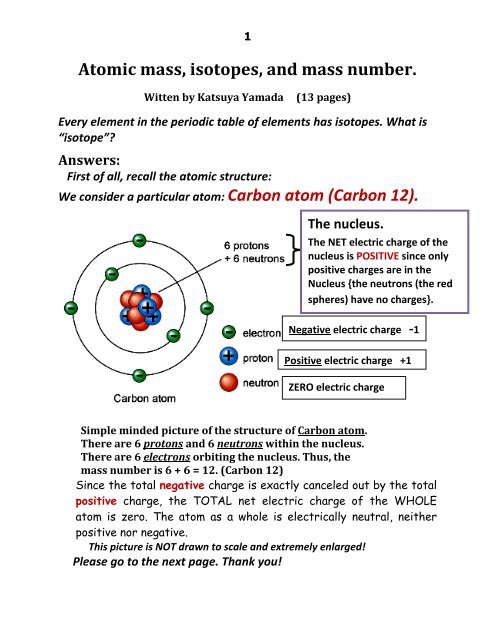
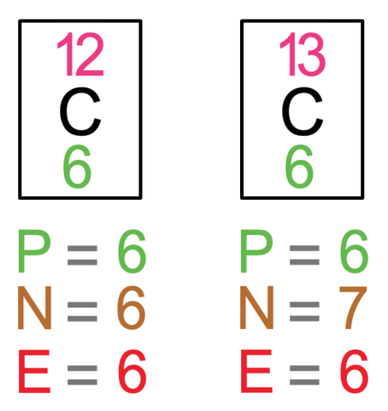
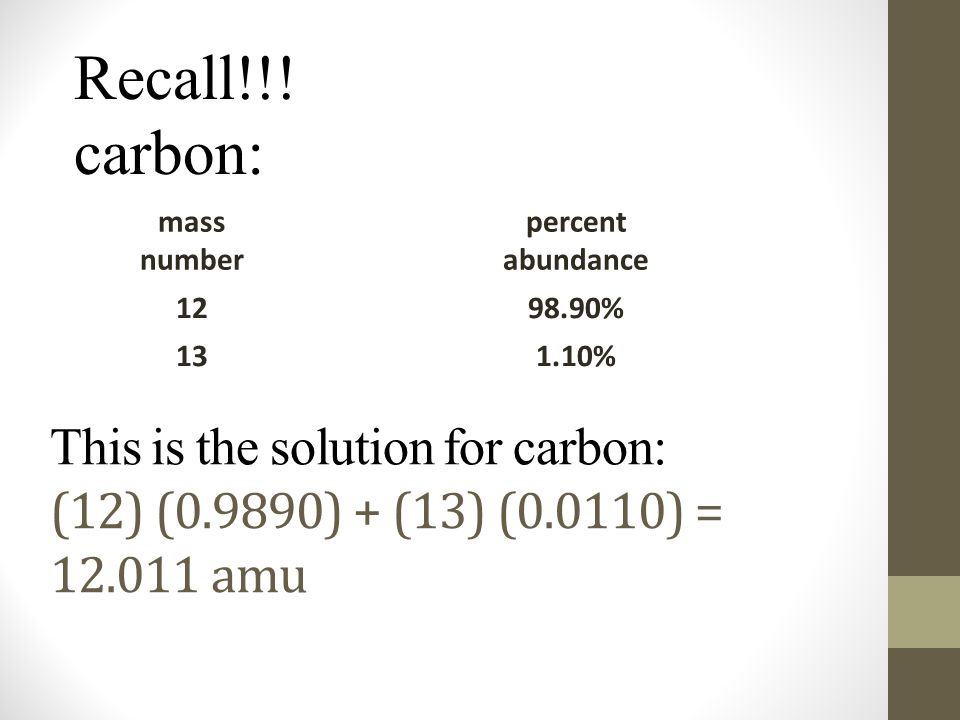ntif we consider that 1/6, in place of 1/12,mass of carbon atom is taken to be relative atomic mass unit ,the mass of 1 mole of substance will?n

if we consider that 1/6, in place of 1/12, mass of carbon atom is taken to be relative atomic mass - Chemistry - Some Basic Concepts of Chemistry - 13772503 | Meritnation.com
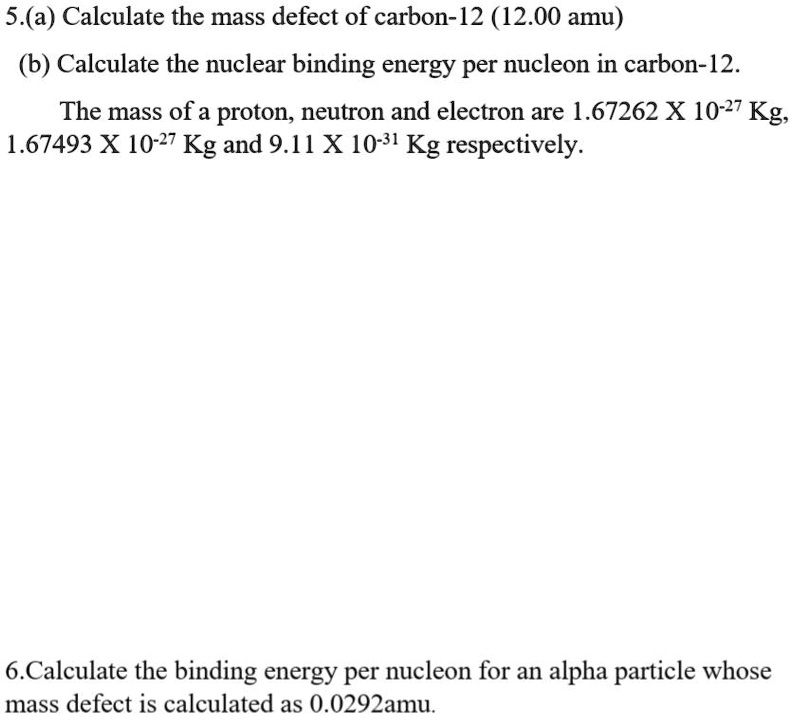
SOLVED: 5.(a) Calculate the mass defect of carbon-12 (12.00 amu) (b) Calculate the nuclear binding energy per nucleon in carbon-12 The mass of a proton, neutron and electron are 1.67262 X 10-27
What is 1/12 of the mass of a carbon 12 atom? And why do we compare atomic masses of other elements with respect to it? - Quora