Limestone is held with tongs and heated on the flame directly. It crumbled to give white powder of calcium oxide. After cooling, when water is added to it a hissing sound is

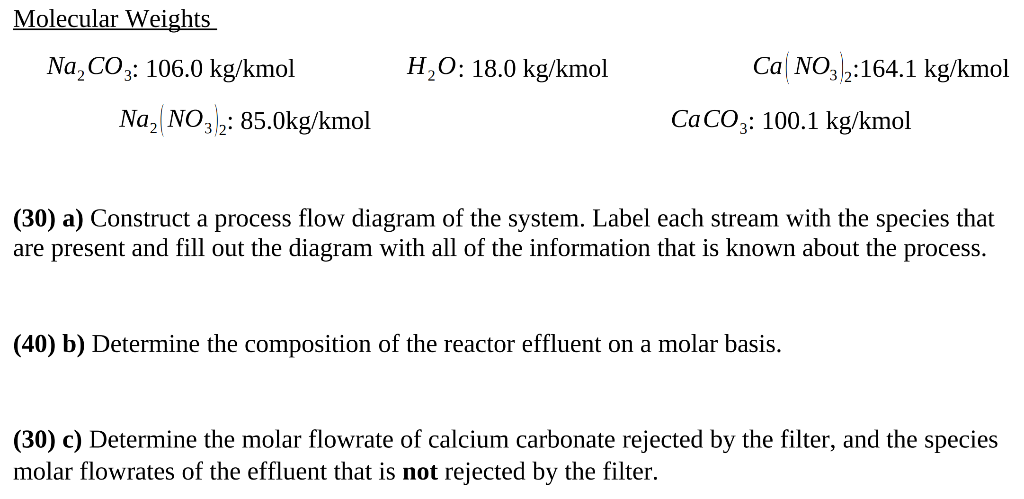
SOLVED: For the reaction Na2CO3+Ca(NO3)2⟶CaCO3+2NaNO3 how many grams of calcium nitrate, Ca(NO3)2, are needed to react completely with 55.5 g of sodium carbonate, Na2CO3?

SOLVED: What will happen if students mix together solutions of calcium nitrate and potassium chloride? Explain your answer: What will happen if students mix together solutions of sodium carbonate and copper(II) nitrate?
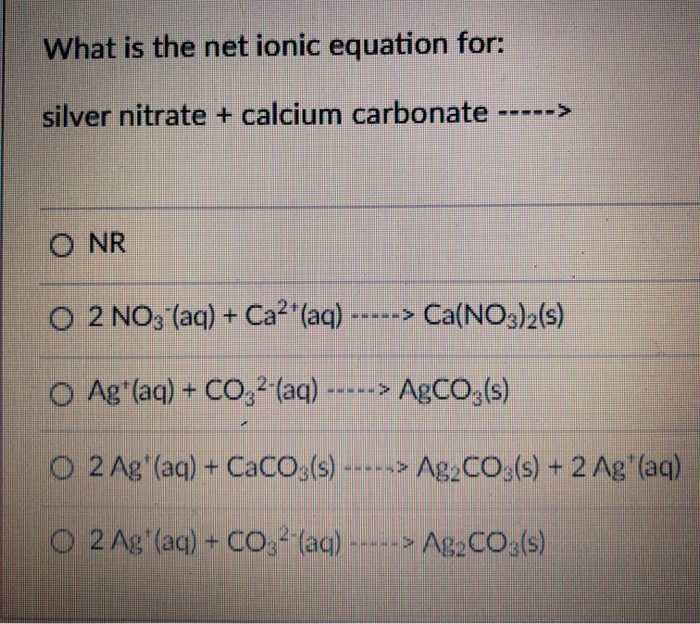
What is the balanced net-ionic equation for the gas producing reaction between hydrobromic acid and solid calcium carbonate? - Quora
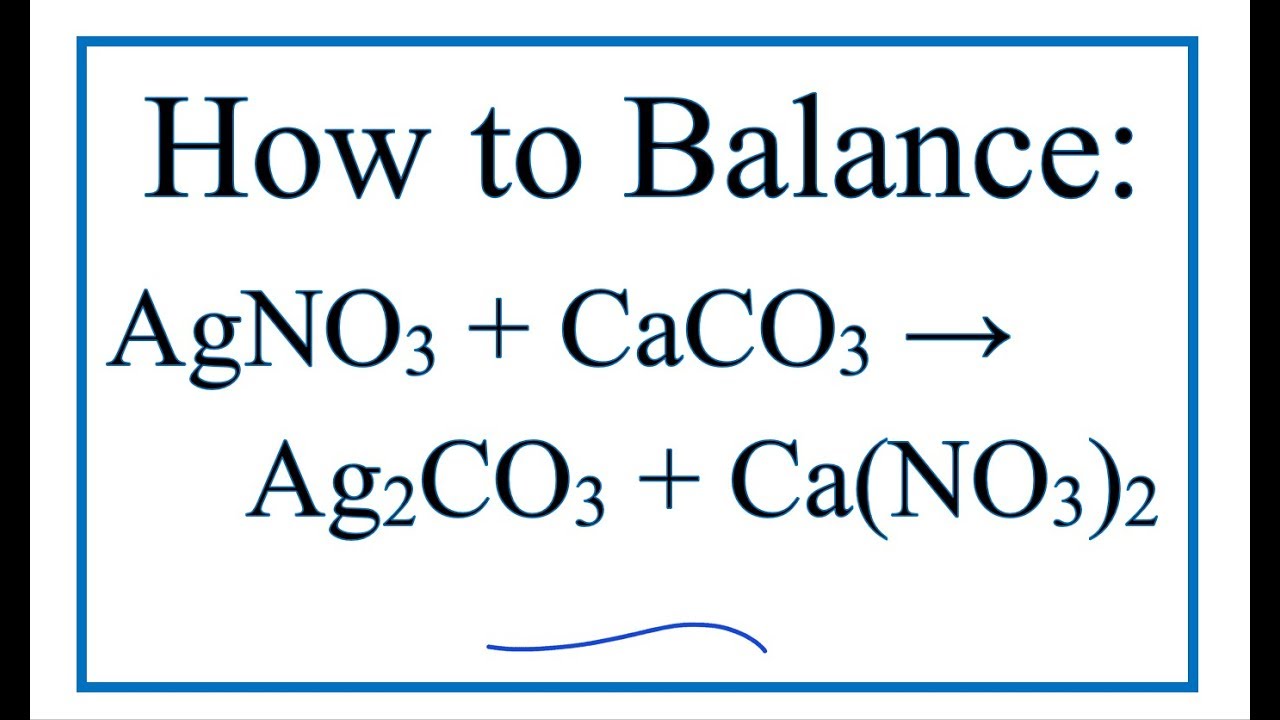
SOLVED: Calcium nitrate reacts with sodium carbonate to precipitate solid calcium carbonate: Ca(NO3)2(aq) + Na2CO3(aq) ? CaCO3(s) + NaNO3(aq) Balance the chemical equation. How many grams of Na2CO3 are needed to react

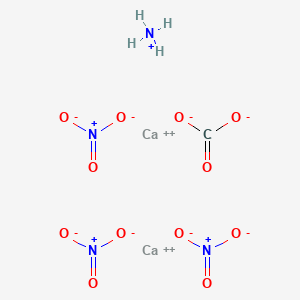
Calcium nitrate reacts with ammonium carbonate to form calcium carbonate and ammonium nitrate . How to - Brainly.in

How to produce calcium nitrate from calcium carbonate? I tried to react calcium carbonates with nitric acid but it didn't work out. Can anyone help me with it - Quora
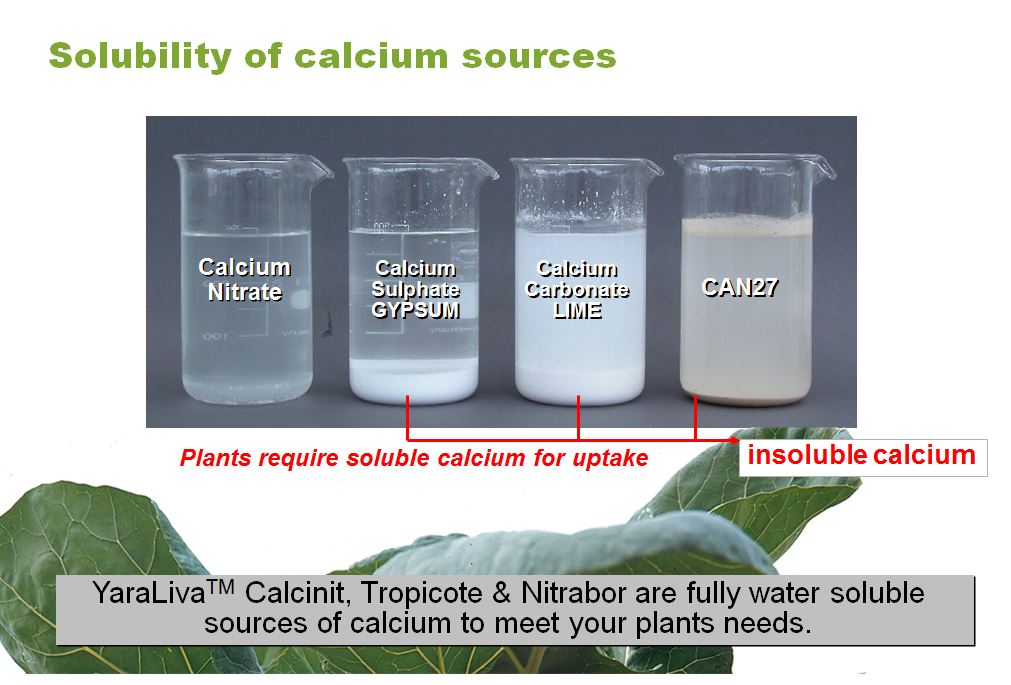
Tracy Beilby on Twitter: "Do you know your Calcium Sources It takes just 1 litre of water to dissolve 1kg of Yara Calcium Nitrate - Fully water soluble calcium that plants need

Factors controlling and influencing polymorphism, morphology and size of calcium carbonate synthesized through the carbonation route: A review - ScienceDirect
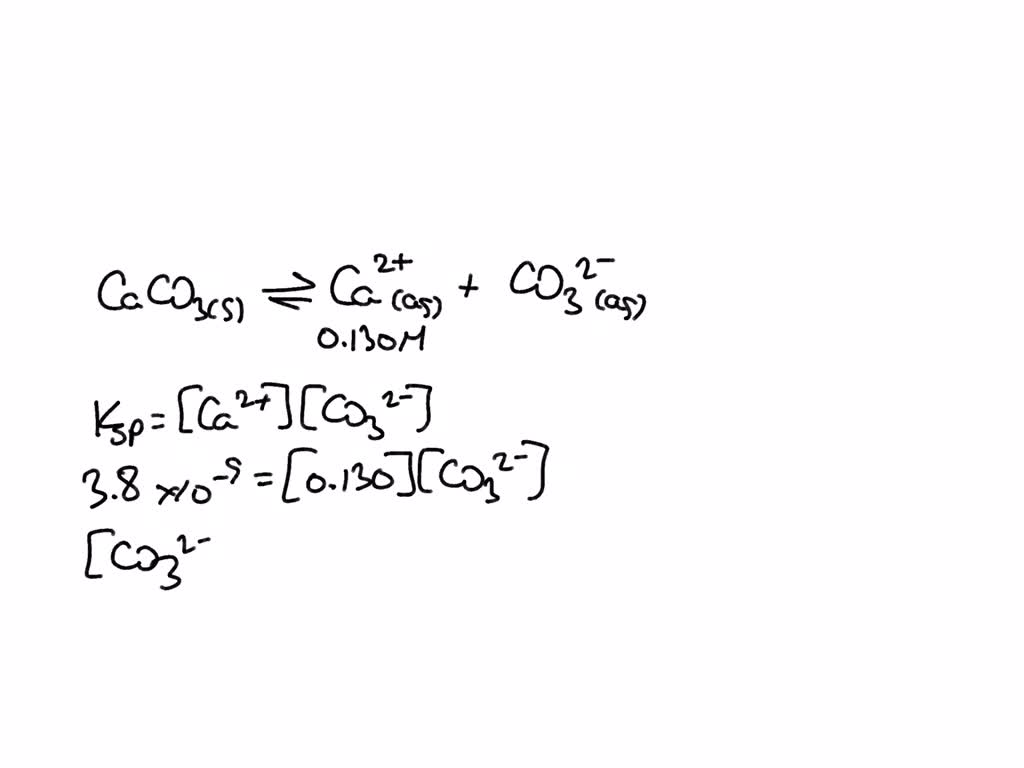
SOLVED: The molar solubility of calcium carbonate in a 0.130 M calcium nitrate solution is M. ksp of CaCO3 is 3.8 × 10-9