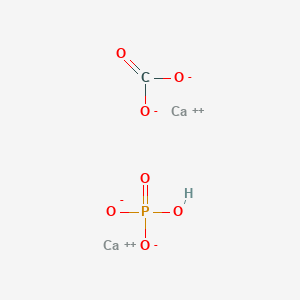
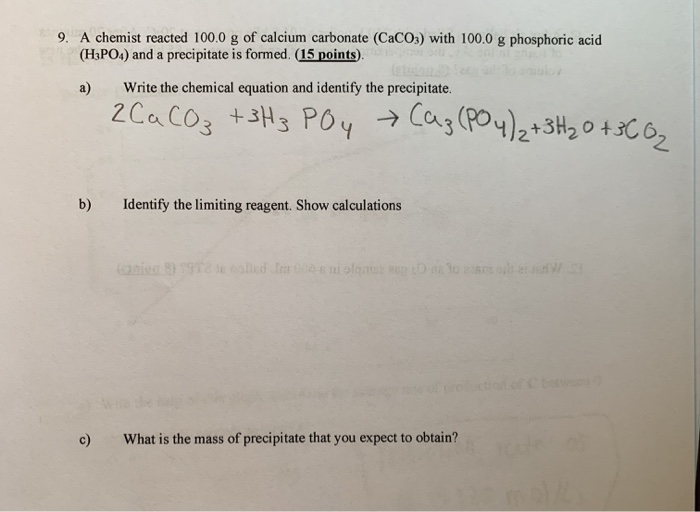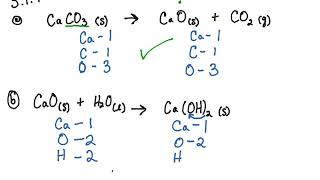Synthesis of calcium hydroxyapatite from calcium carbonate and different orthophosphate sources: A comparative study - ScienceDirect

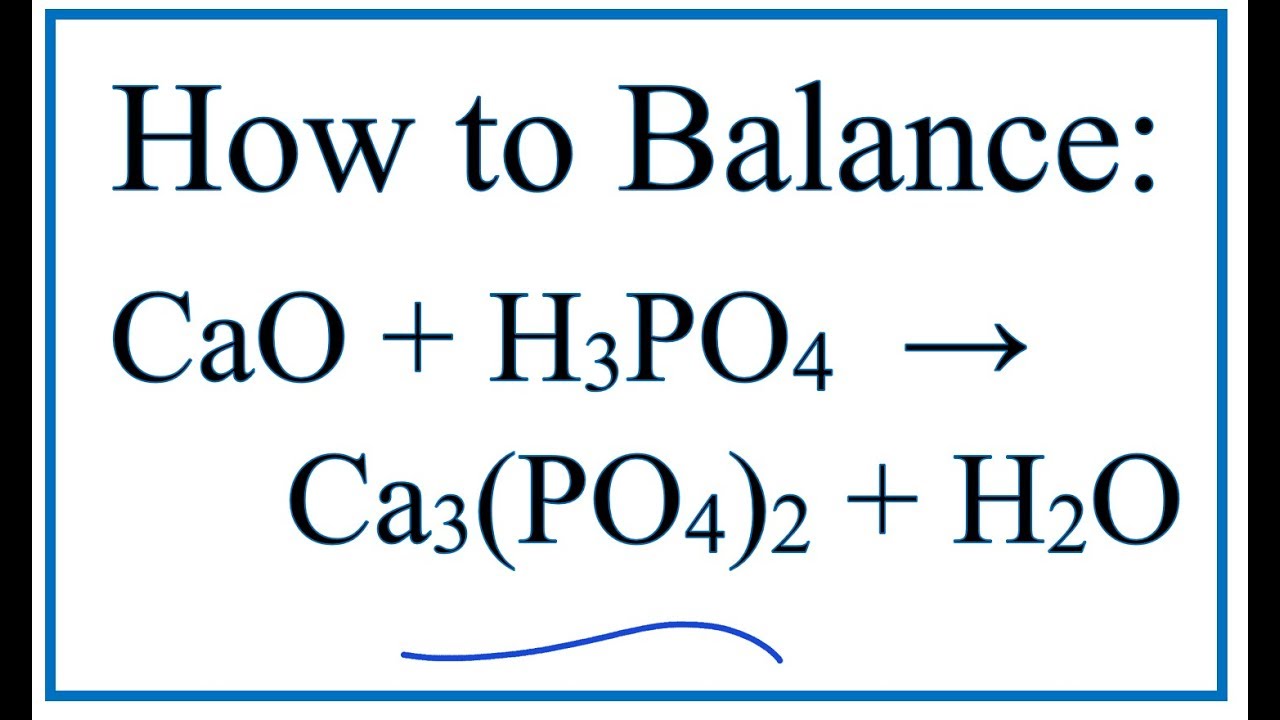
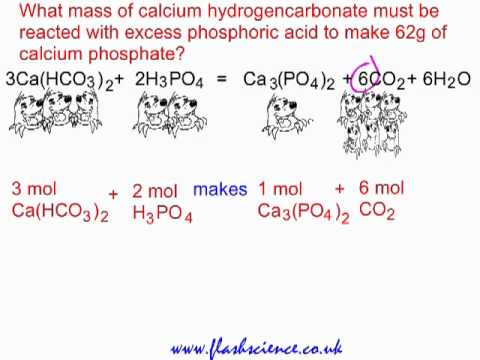
SOLVED:Write balanced chemical equations for each of the following processes: (a) Calcium phosphate reacts with sulfuric acid to produce calcium sulfate and phosphoric acid. (b) Calcium phosphate reacts with water containing dissolved

Factors controlling and influencing polymorphism, morphology and size of calcium carbonate synthesized through the carbonation route: A review - ScienceDirect

Urease-aided calcium carbonate mineralization for engineering applications: A review - ScienceDirect
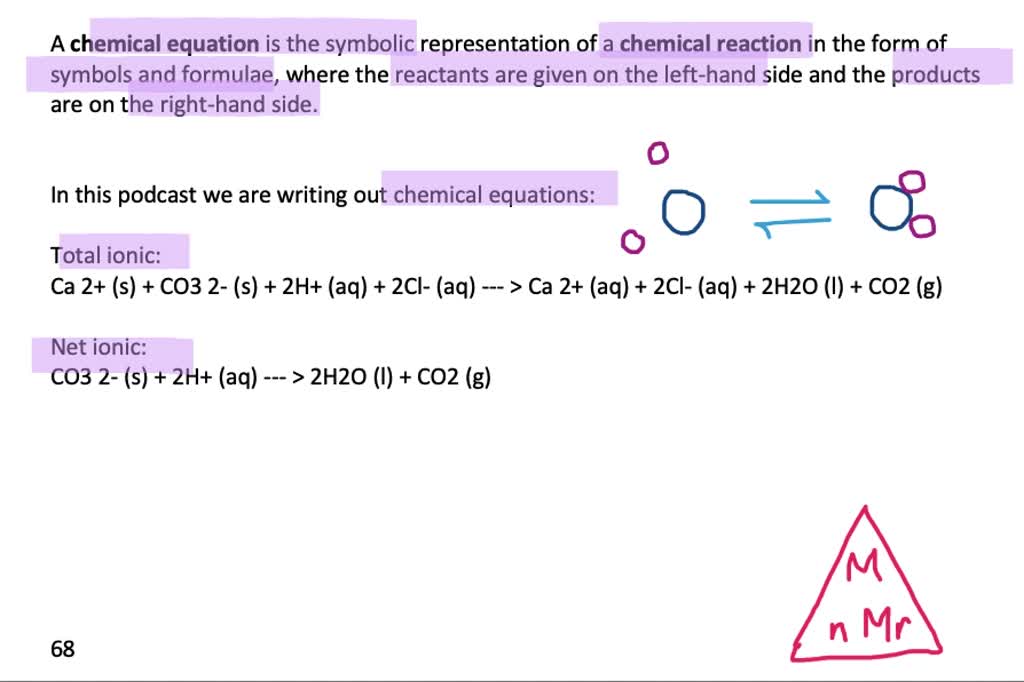
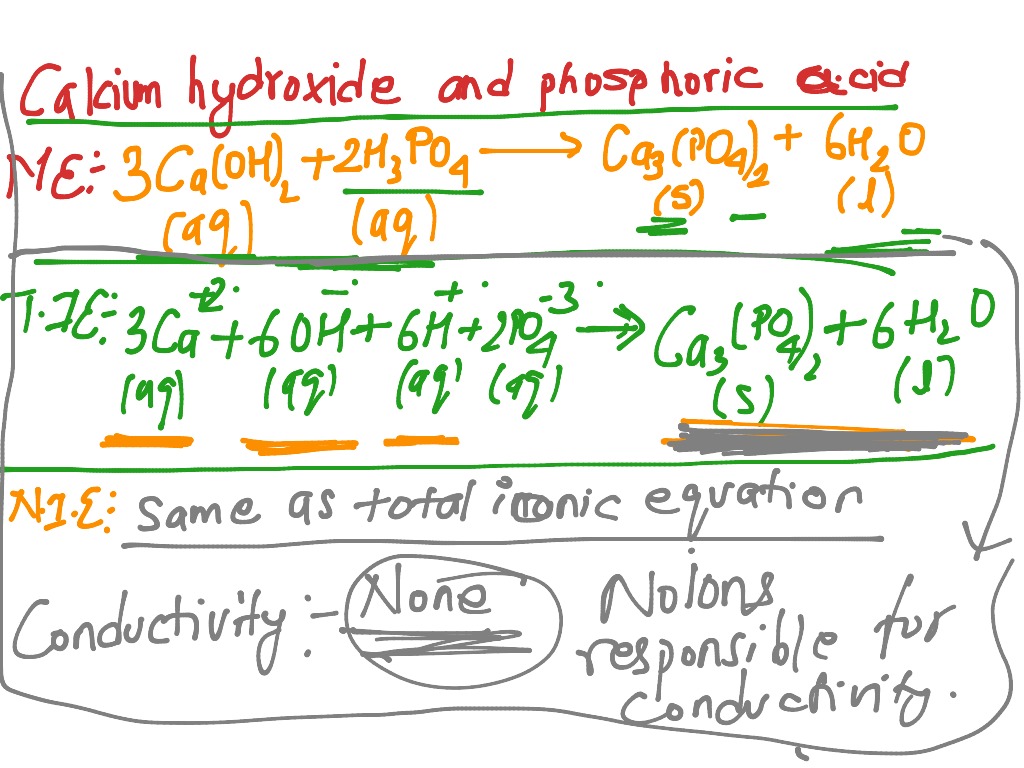
SOLVED: In the stomach, both the carbonate and phosphate ions react with the hydrochloric acid (HCl) in your stomach to become carbonic and phosphoric acid. When this happens, the calcium and chloride
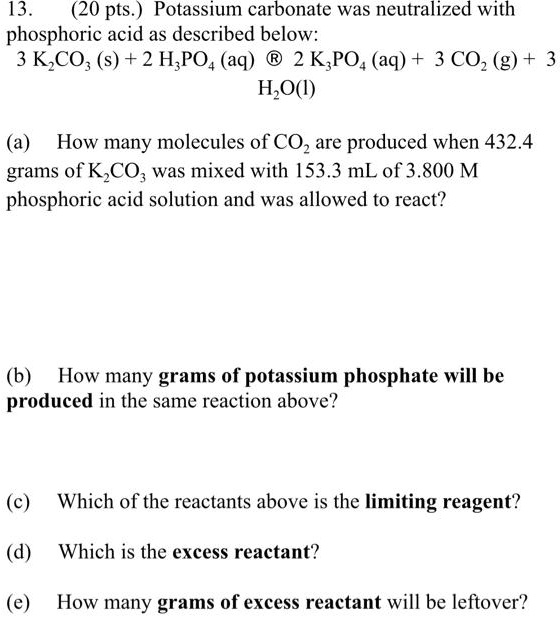
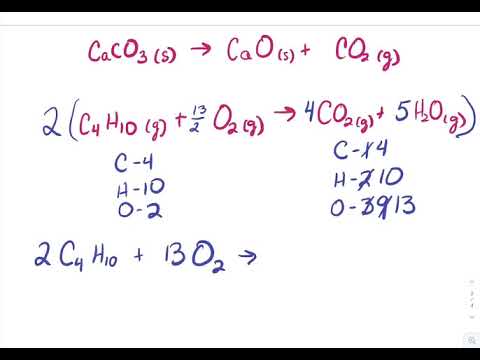
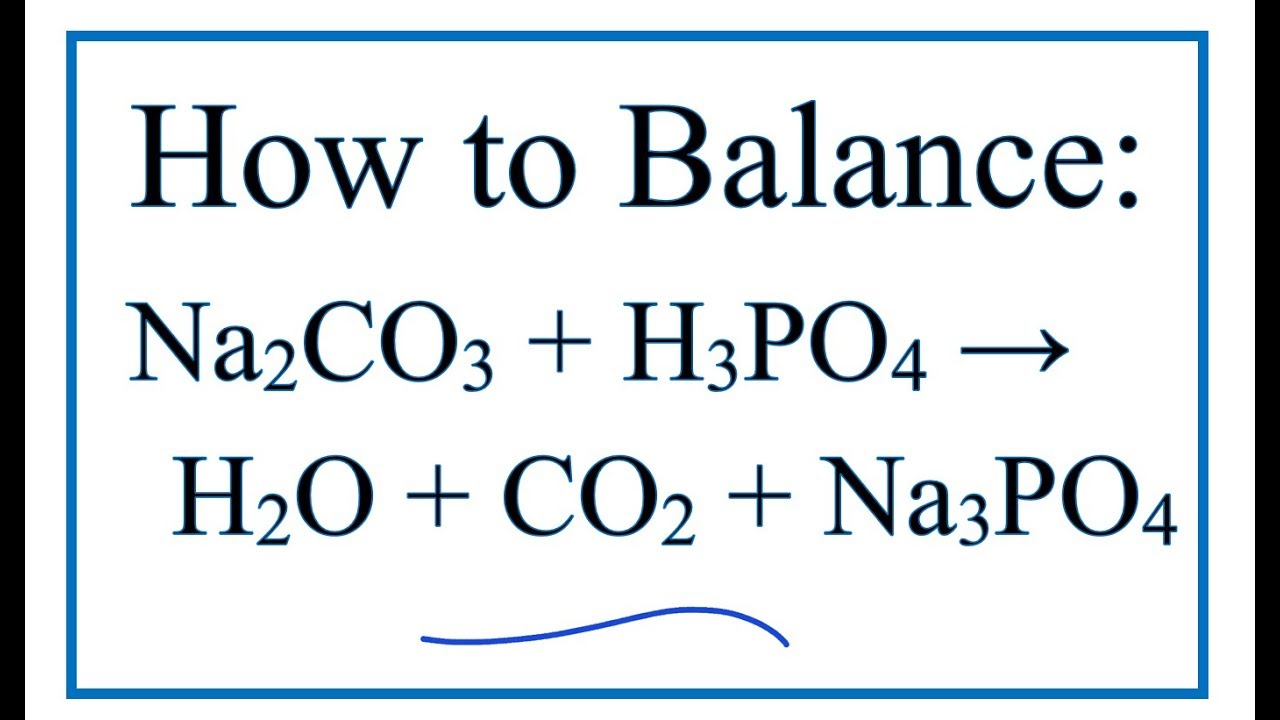
SOLVED: 13 (20 pts Potassium carbonate was neutralized with phosphoric acid as described below: KCO; (s) + 2 H;PO4 (aq) 2 K;PO4 (aq) + 3 COz (g) + H,O() (a) How many

ChemEngineering | Free Full-Text | Hydrothermal Synthesis of Biphasic Calcium Phosphate from Cuttlebone Assisted by the Biosurfactant L-rhamnose Monohydrate for Biomedical Materials