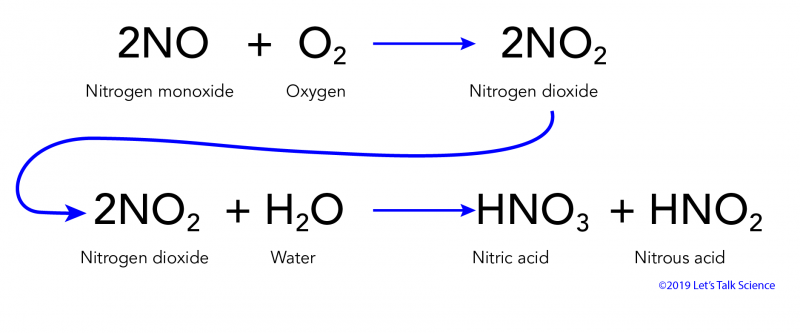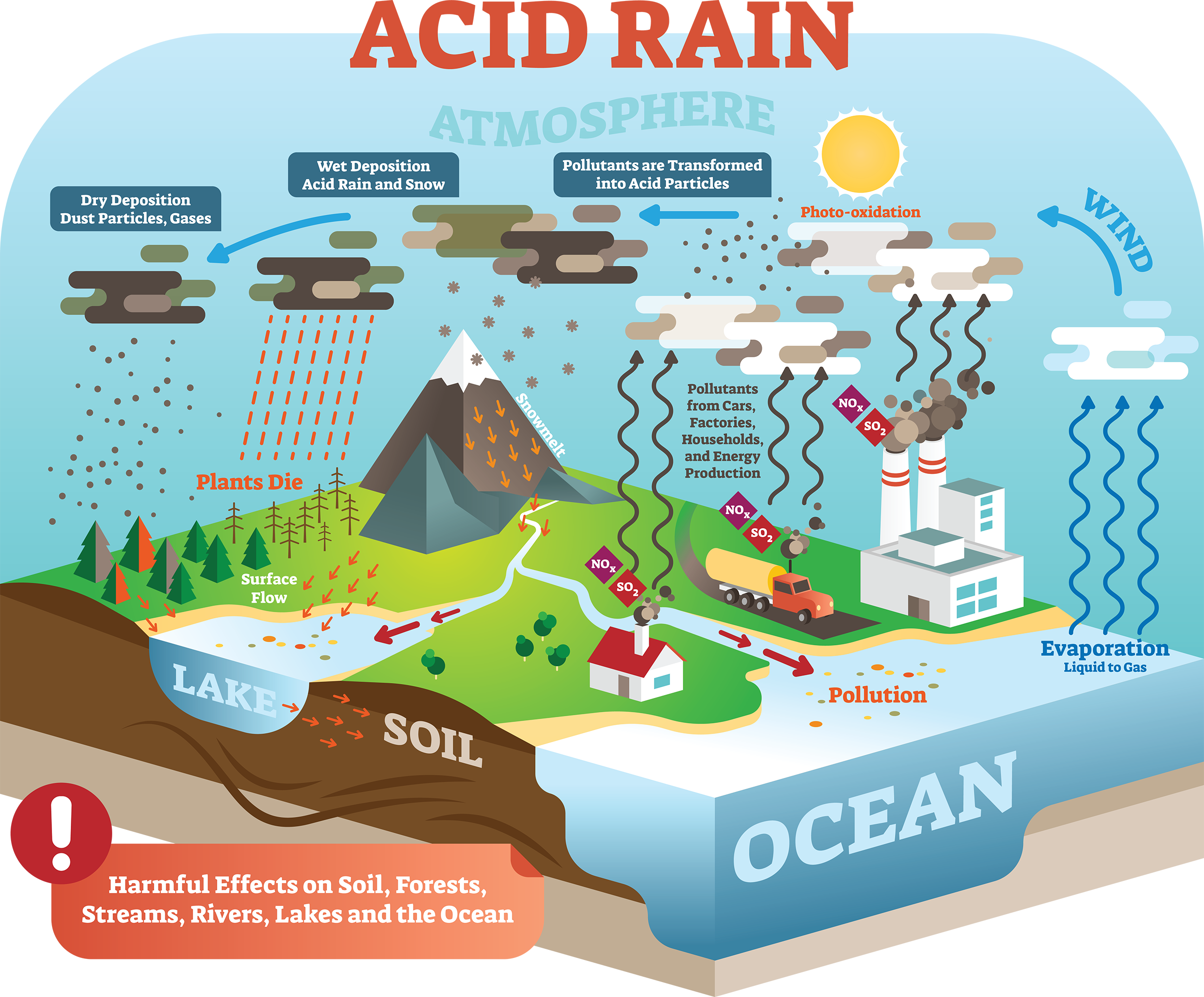
CWK CWK Acid Rain State the adverse effect of these common pollutants on buildings and why these pollutants are of global concern Relate the effects. - ppt download

Application of microbially induced calcium carbonate precipitation with urea hydrolysis to improve the mechanical properties of soil - ScienceDirect
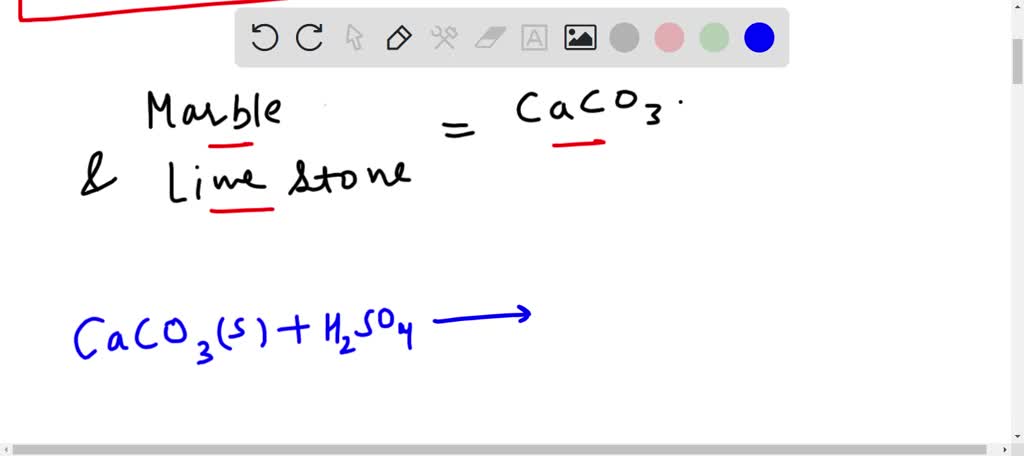
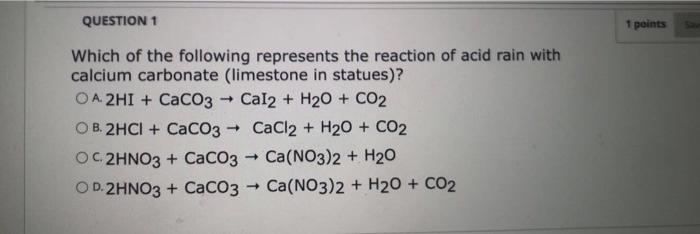
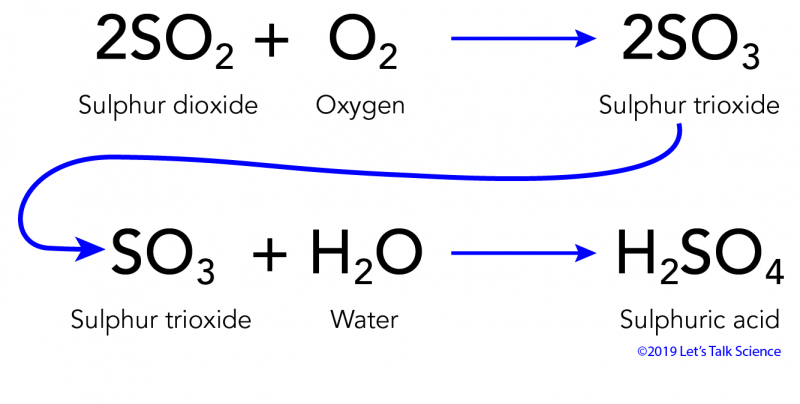
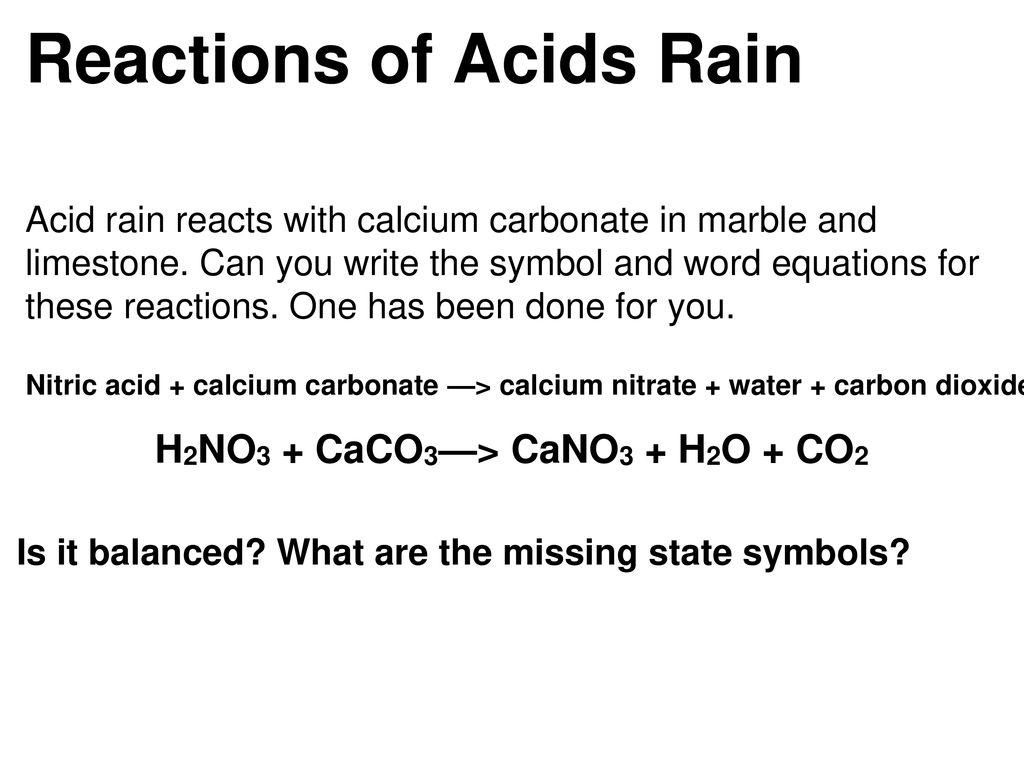
SOLVED: Write an equation to show how sulfuric acids in acid rain reacts with marble and limestone. (Both marble and limestone are primarily calcium carbonate.)
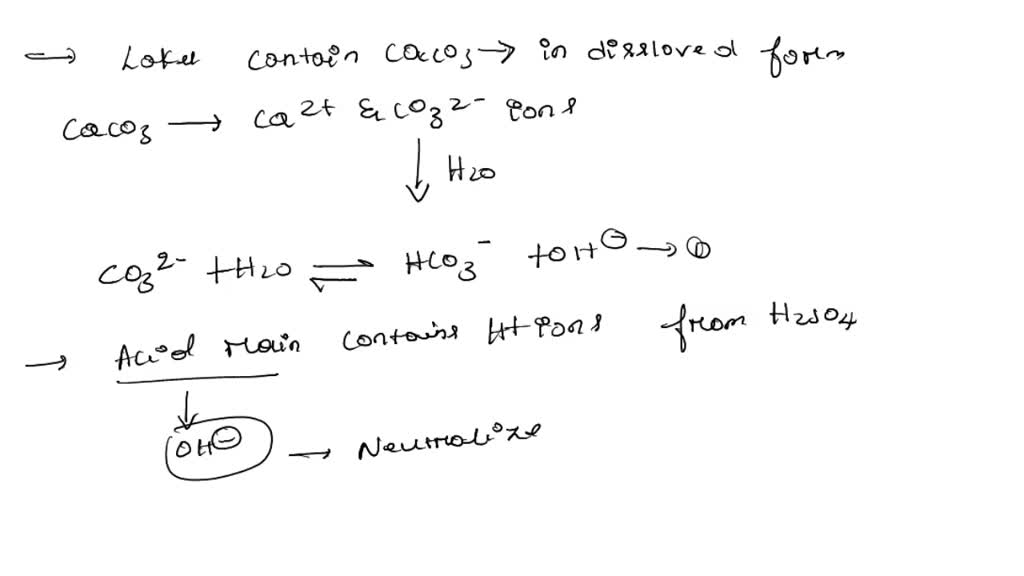
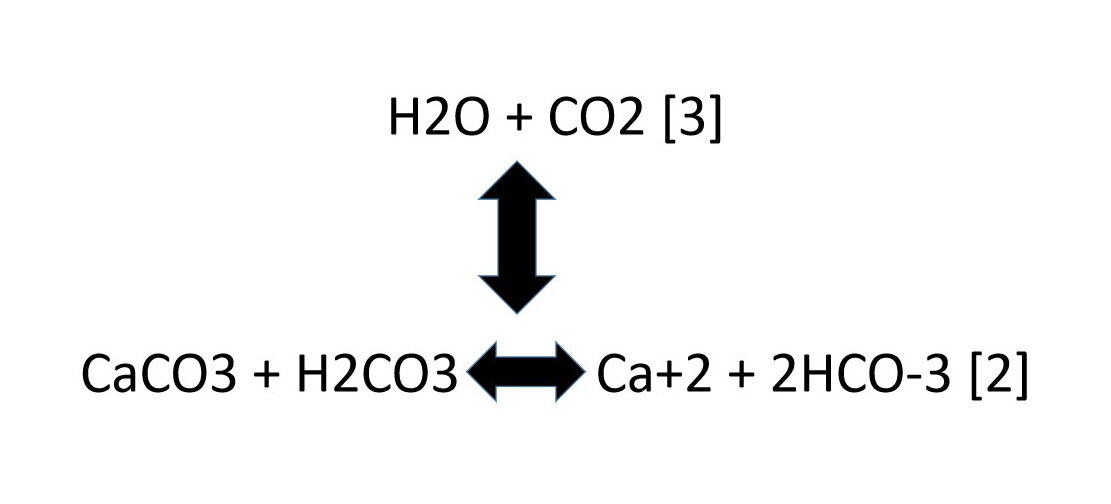
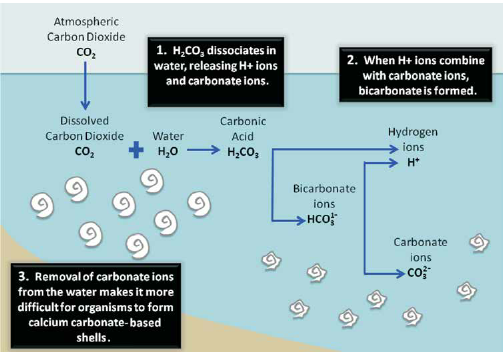
SOLVED: Lakes have a natural buffering capacity, especially in regions where limestone gives rise to dissolved calcium carbonate. Write an equation for the effect of a small amount of acid rain containing

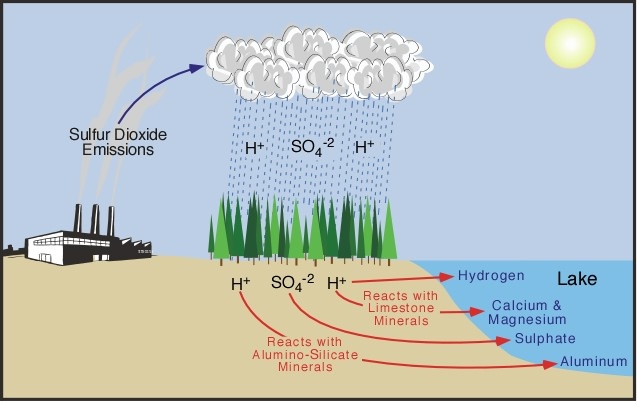
Where does our drinking water come from? This means the water we drink has run through and across rocks. - ppt download
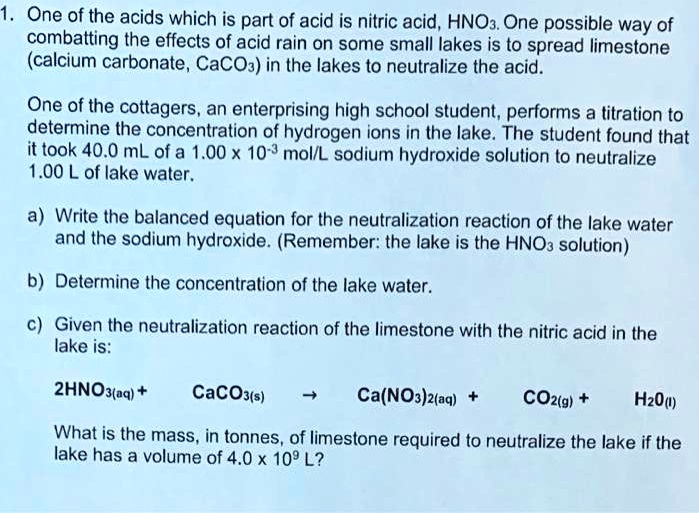
SOLVED: One of the acids which is part of acid is nitric acid, HNO: One possible way of combatting the effects of acid rain on some smali lakes is 0 spread limestone (