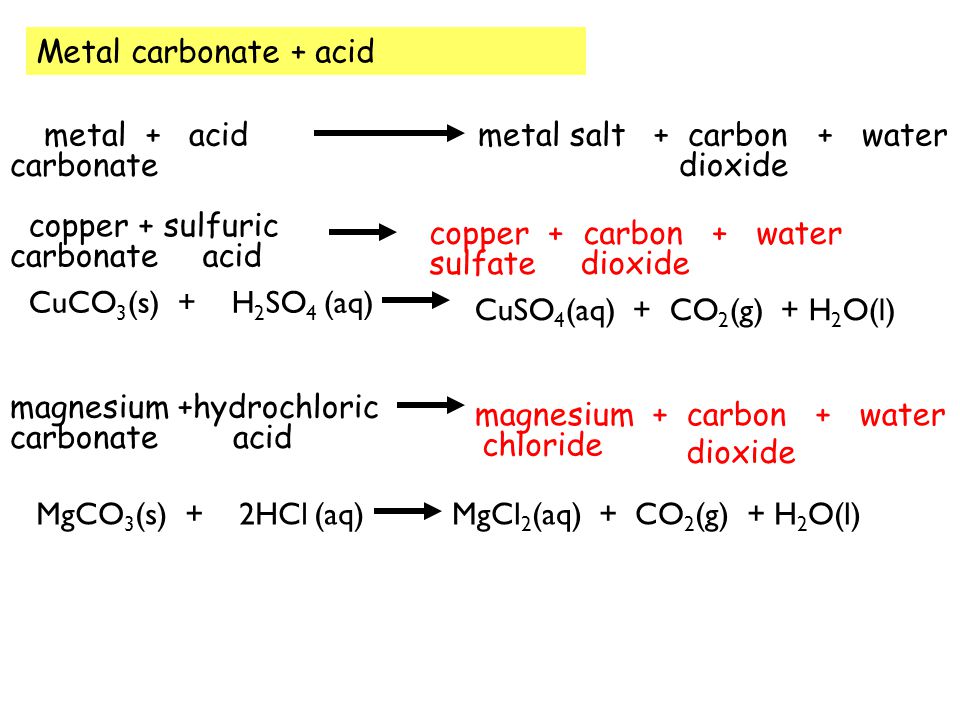
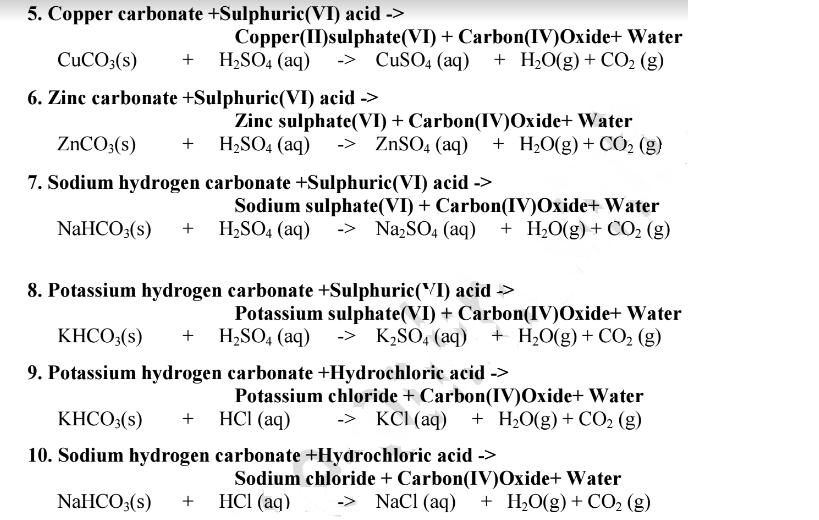
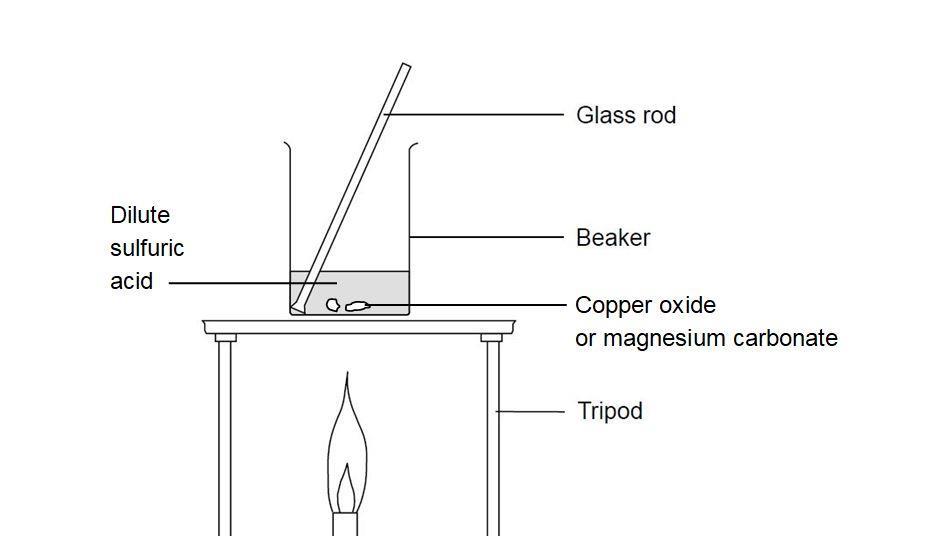
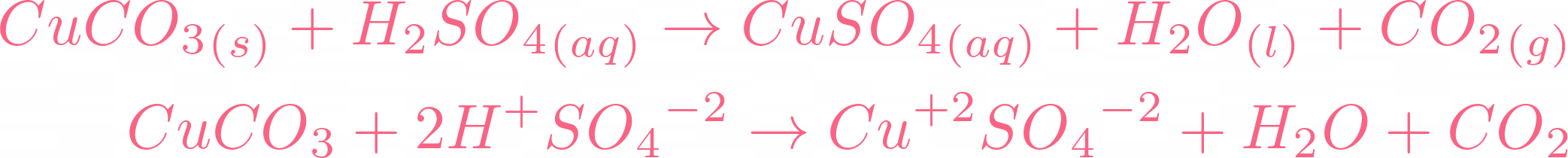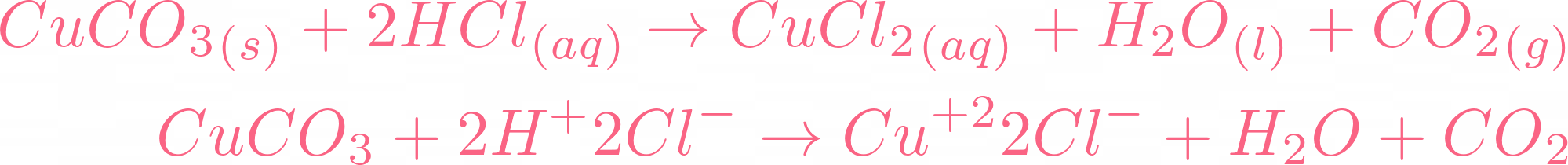SOLVED: Copper(II) sulfate crystals are made by reacting copper(II) carbonate with dilute sulfurc acid . The equation for the overall process shown Cuco, H,SO4 4H,0 Cuso 5H,O CO; step Powdered solid copper(II)

Most carbonates are insoluble (can not be dissolved in water) except those containing sodium or potassium ions. - ppt download
![Chemical Reactions to make copper(II) sulfate| Chemistry Basics [Online Video] – O Level Secondary Chemistry Tuition Chemical Reactions to make copper(II) sulfate| Chemistry Basics [Online Video] – O Level Secondary Chemistry Tuition](https://icandochemistry942105908.files.wordpress.com/2021/05/mq2-3.jpg?w=640)
Chemical Reactions to make copper(II) sulfate| Chemistry Basics [Online Video] – O Level Secondary Chemistry Tuition
![Chemical Reactions to make copper(II) sulfate| Chemistry Basics [Online Video] – O Level Secondary Chemistry Tuition Chemical Reactions to make copper(II) sulfate| Chemistry Basics [Online Video] – O Level Secondary Chemistry Tuition](http://img.youtube.com/vi/9tJGRn8Su8s/0.jpg)
Chemical Reactions to make copper(II) sulfate| Chemistry Basics [Online Video] – O Level Secondary Chemistry Tuition