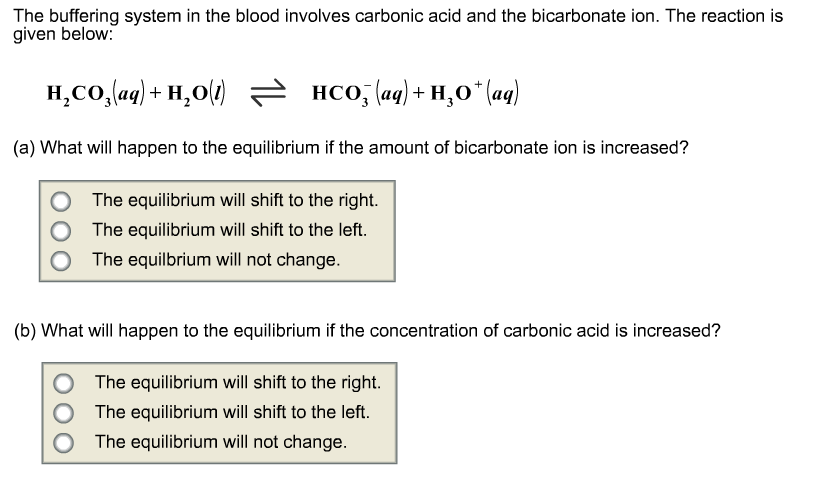
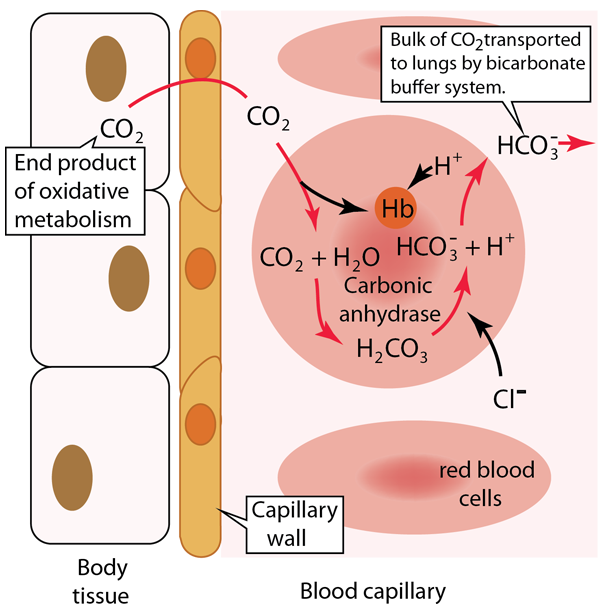
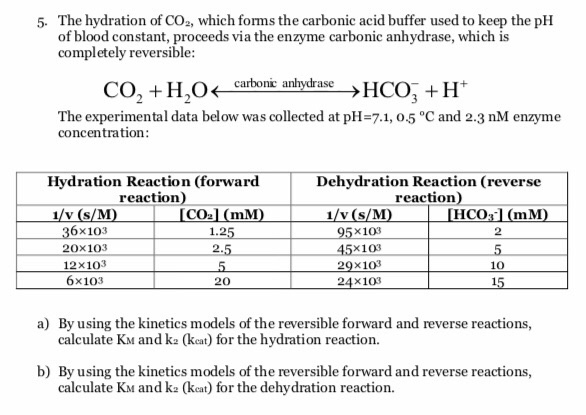
SOLVED: Question 34 Pis Carbonic acid (H2CO3) serves as buffer in human blood Carbonic acid is weak acid that dissociates into bicarbonate ion (HCO3 and hydrogen ion (H*) Thus HzCO3 HCO3" Ht
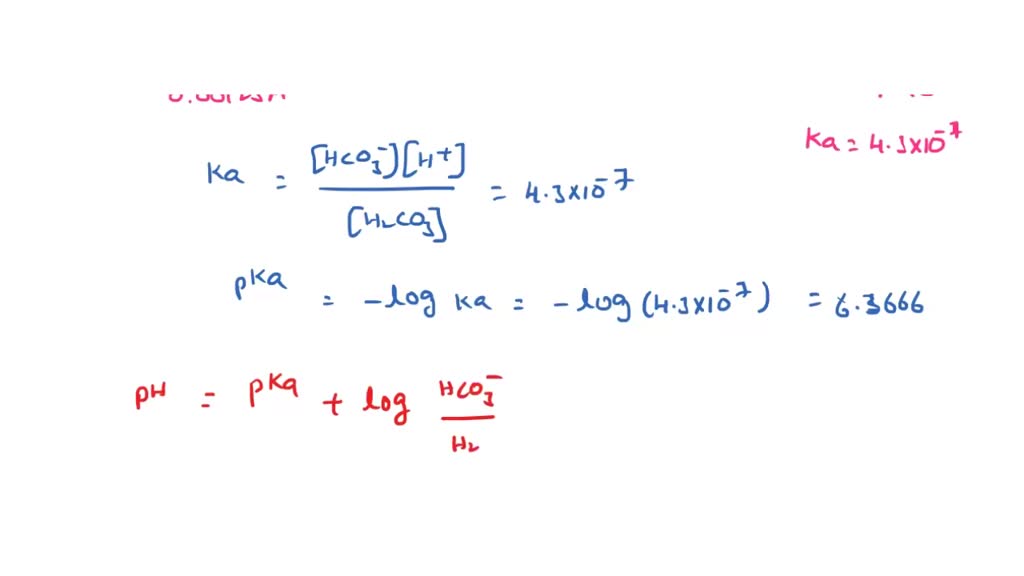
SOLVED: If the carbonic acid (H2CO3) concentration in a sample of blood is 0.00125 M, determine the bicarbonate ion (HCO3-) concentration required to buffer the pH of blood at pH = 7.40.
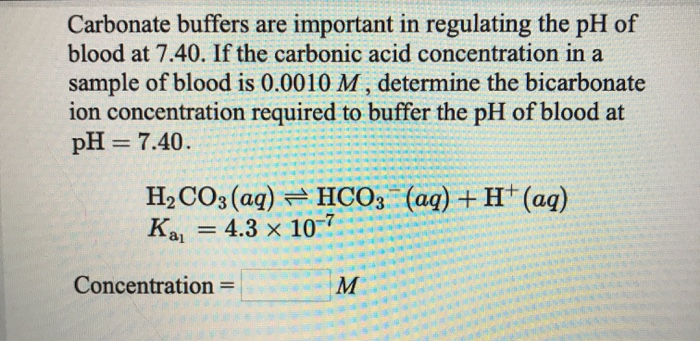
✓ Solved: Carbonate buffers are important in regulating the pH of blood at 7.40.If the carbonic acid...
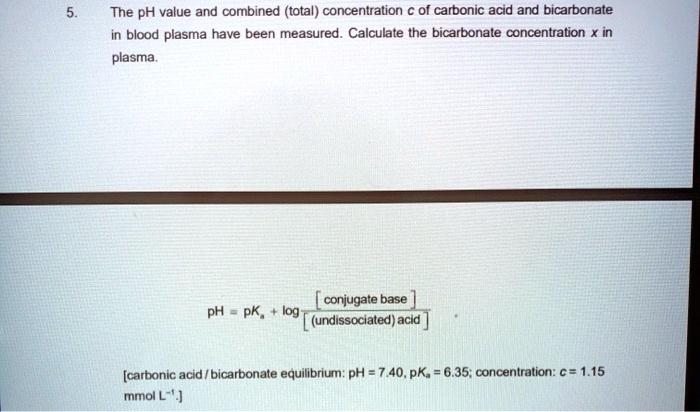
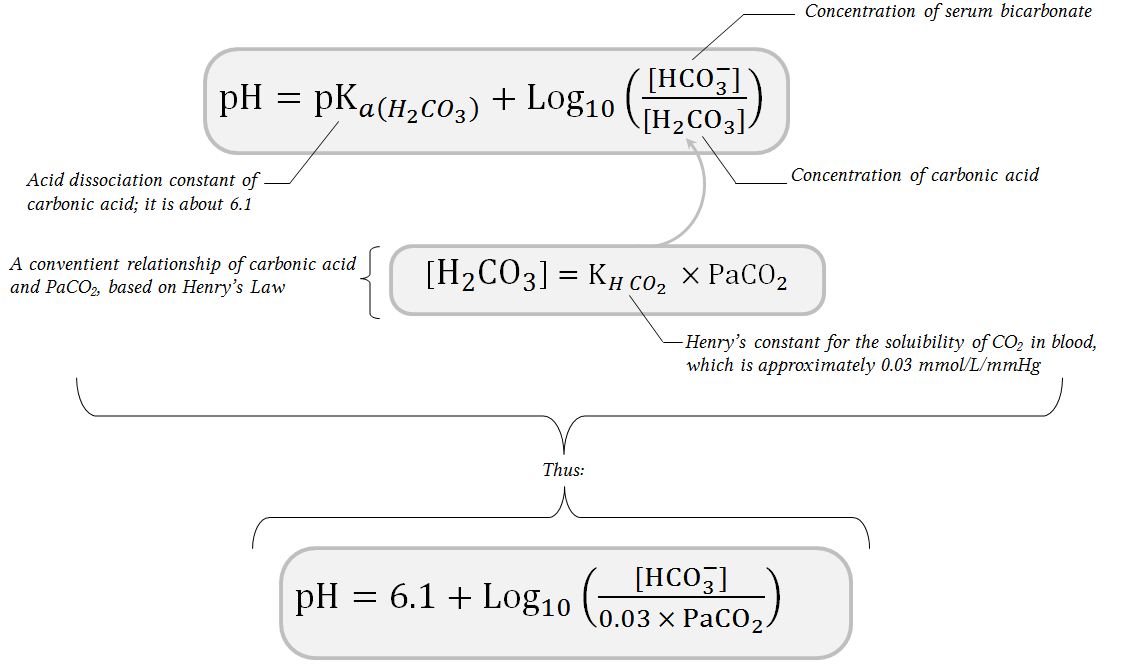
SOLVED: The pH value and combined (total) concentration of carbonic acid and bicarbonate in blood plasma have been measured Calculate the bicarbonate concentration X in plasma conjugate base pH pK log (undissociated)
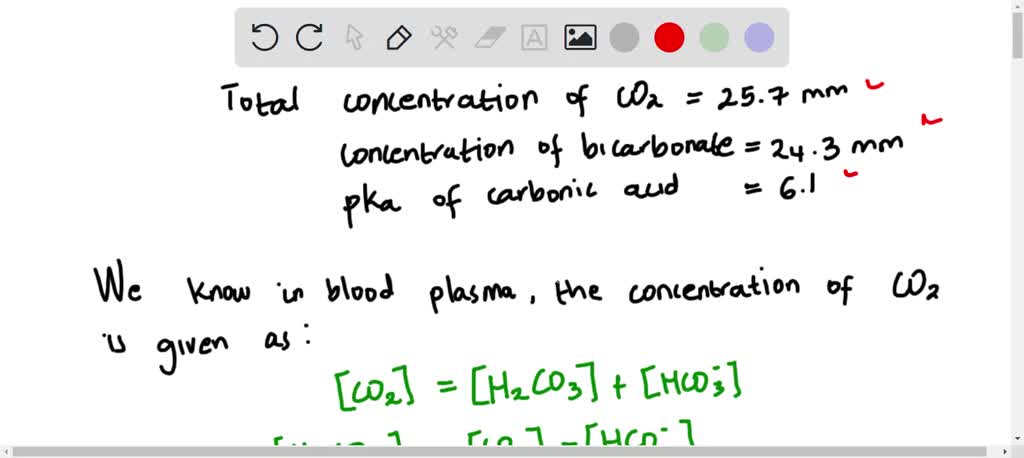
SOLVED: Calculate the pH of a blood plasma sample with a total CO2CO2 concentration of 25.7 mM25.7 mM and bicarbonate concentration of 24.3 mM.24.3 mM. The relevant p𝐾apKa of carbonic acid is