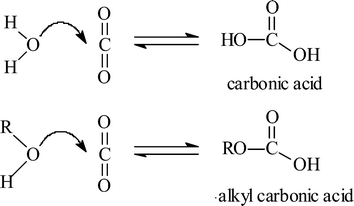
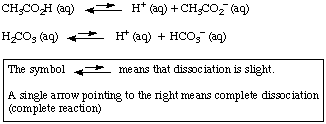Carbonic Acid: An Important Intermediate in the Surface Chemistry of Calcium Carbonate | Journal of the American Chemical Society

Neutralization Reaction Definition ,Equation ,Examples and Facts – Chemistry - Best Online Free Chemistry Learning

Question Video: Determining the Products of the Neutralization Reaction of Barium Hydroxide Ba(OH)₂ with Carbonic Acid H₂CO₃ | Nagwa

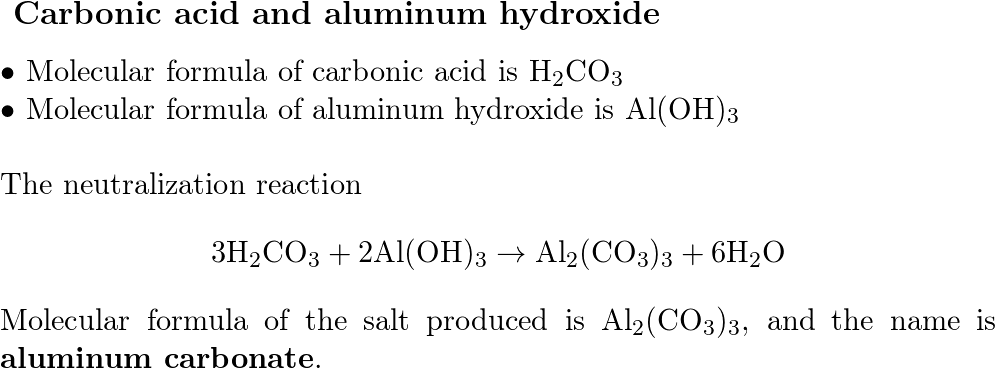
Question: Write the chemical reaction when lithium hydroxide is mixed with carbonic acid. Step 1: write out the reactants LiOH (aq) + H 2 CO 3 (aq) - ppt download
Visualization of the shortest routes for the decomposition of isolated... | Download Scientific Diagram

Carbonic Acid: An Important Intermediate in the Surface Chemistry of Calcium Carbonate | Journal of the American Chemical Society
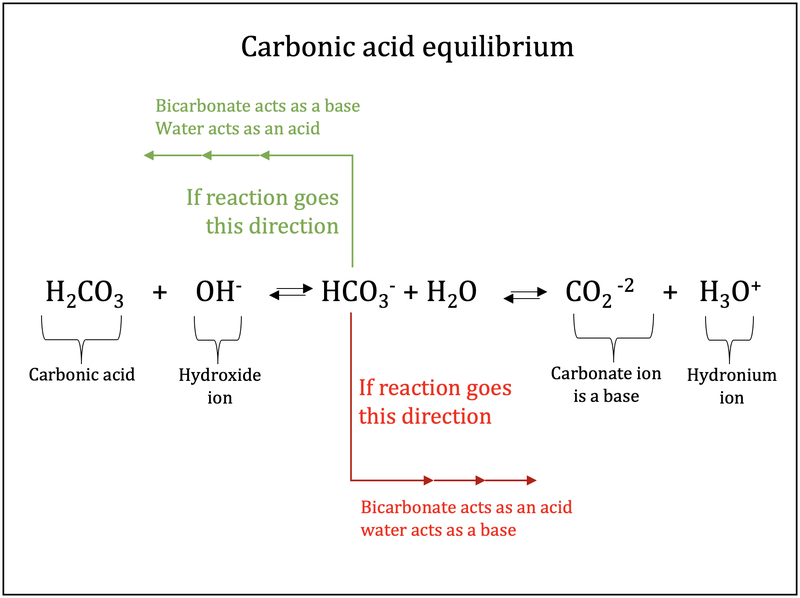
Why is carbonic acid a weak acid even though it gets completely dissociated into H+ and CO3- ions? - Quora

Carbonic Acid: An Important Intermediate in the Surface Chemistry of Calcium Carbonate | Journal of the American Chemical Society

Drill #12 5/13, 14/2014 Write the balanced neutralization equations for the following reactions: 1. carbonic acid & calcium hydroxide 2. potassium hydroxide. - ppt download

Question: Write the chemical reaction when lithium hydroxide is mixed with carbonic acid. Step 1: write out the reactants LiOH (aq) + H 2 CO 3 (aq) - ppt download