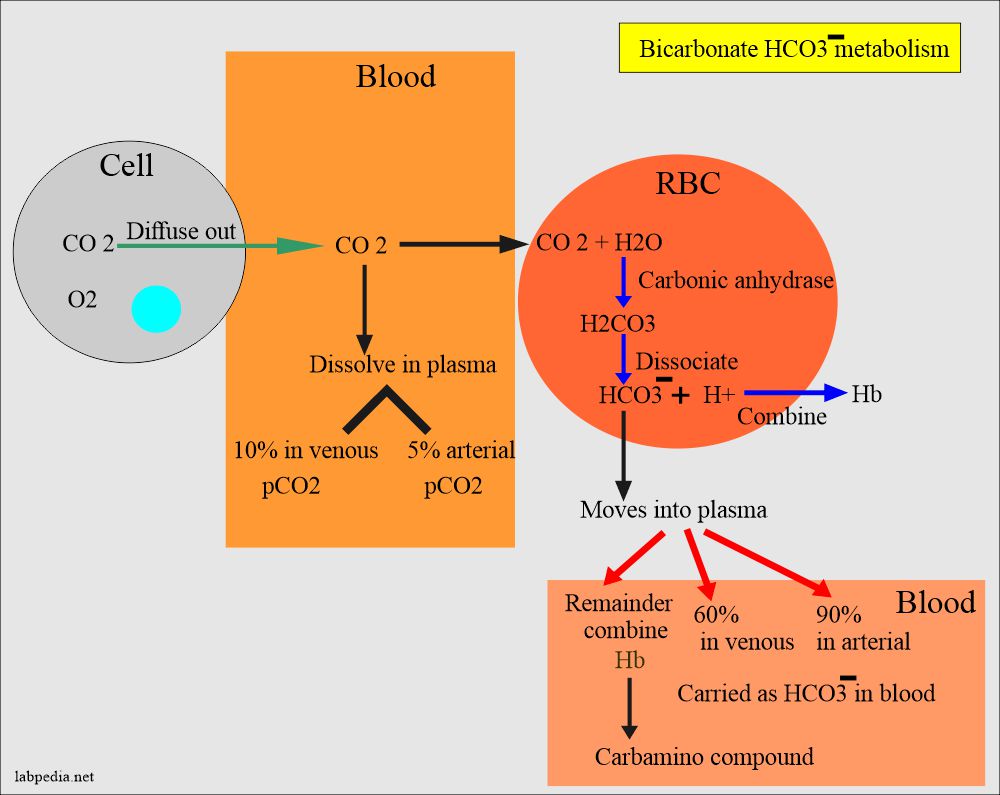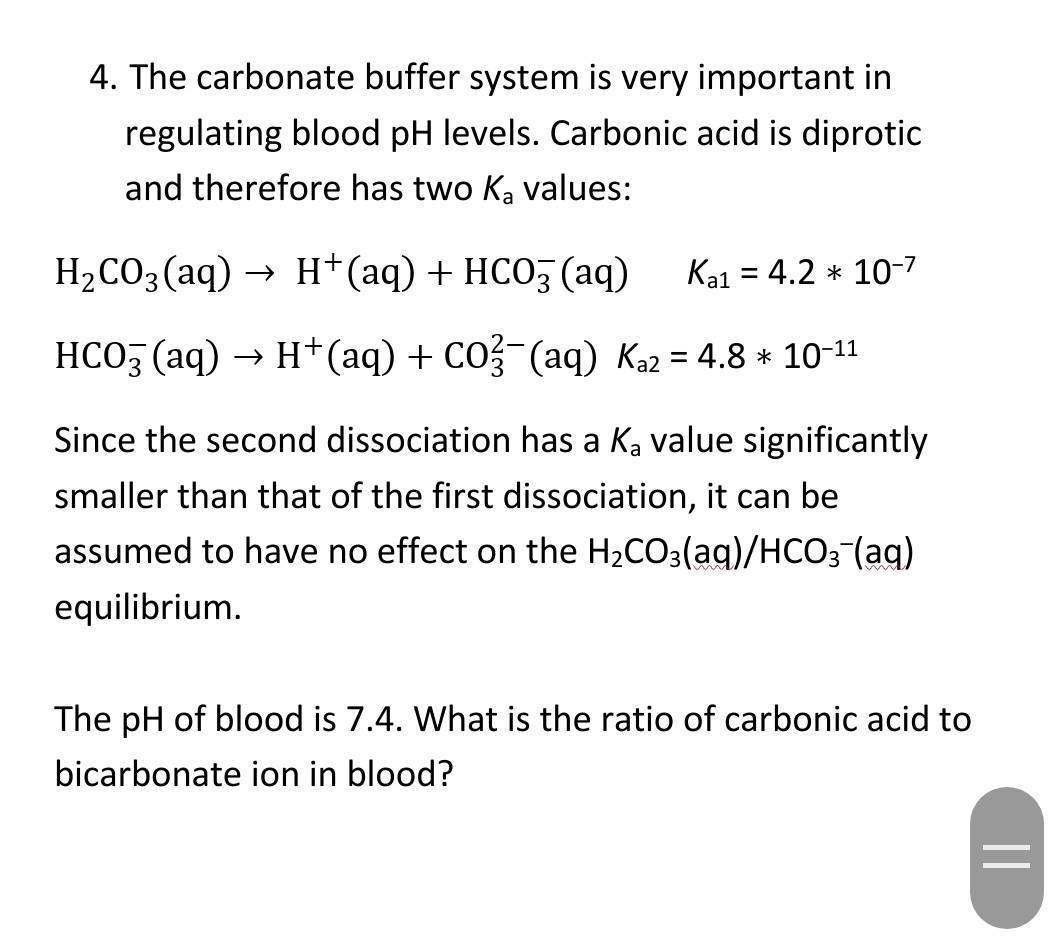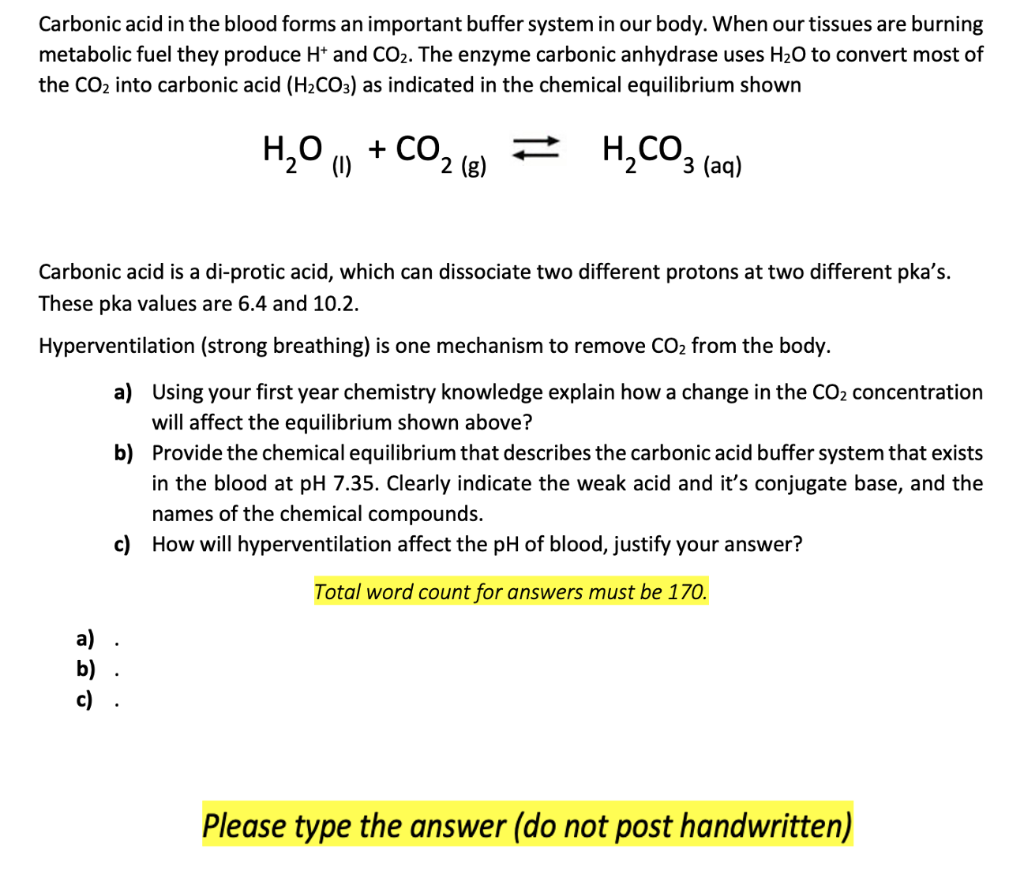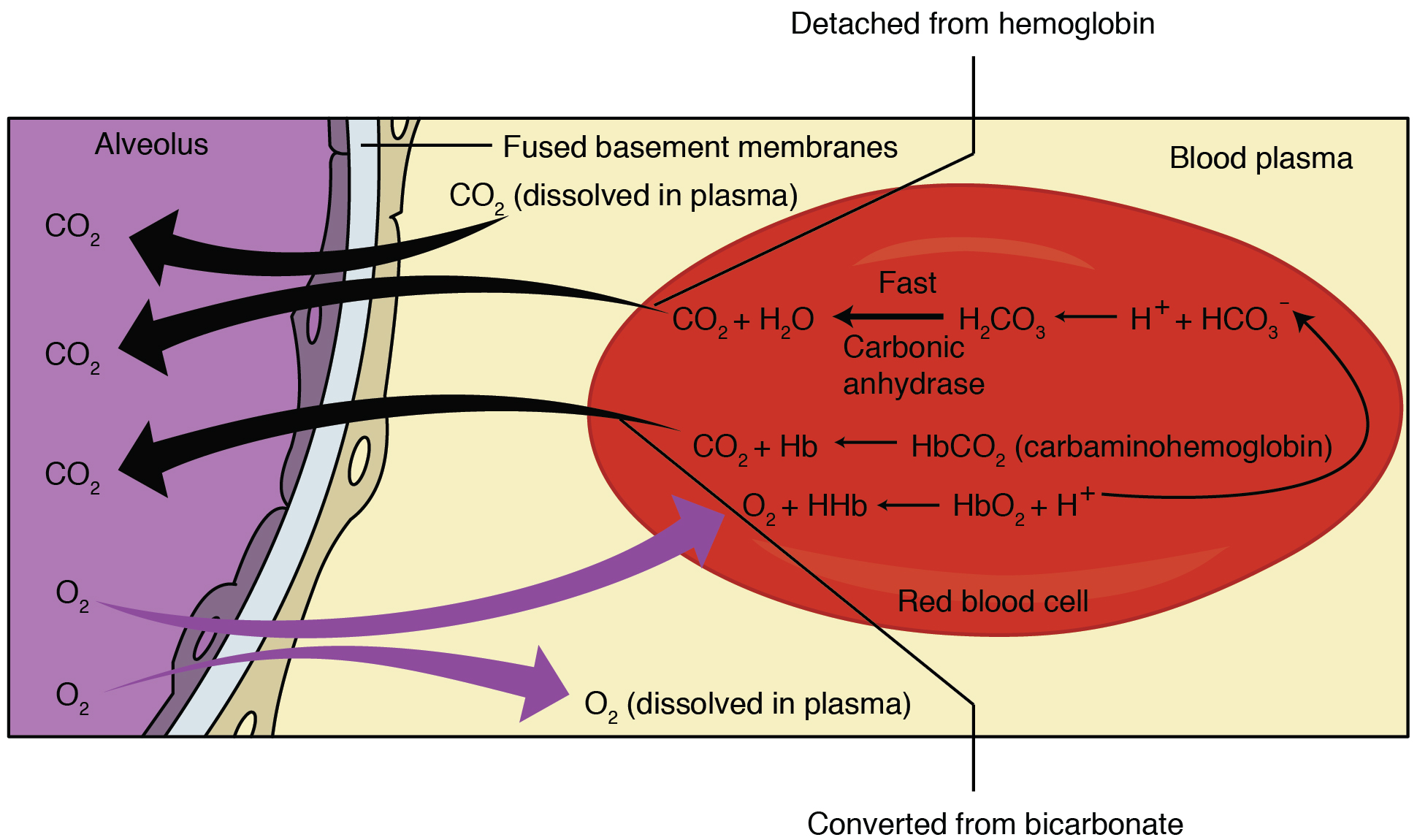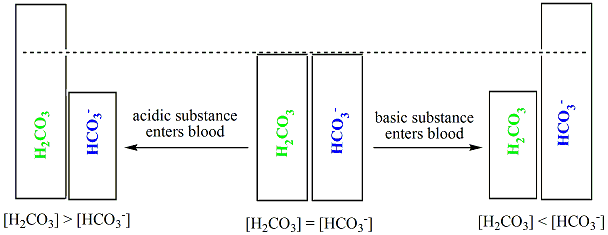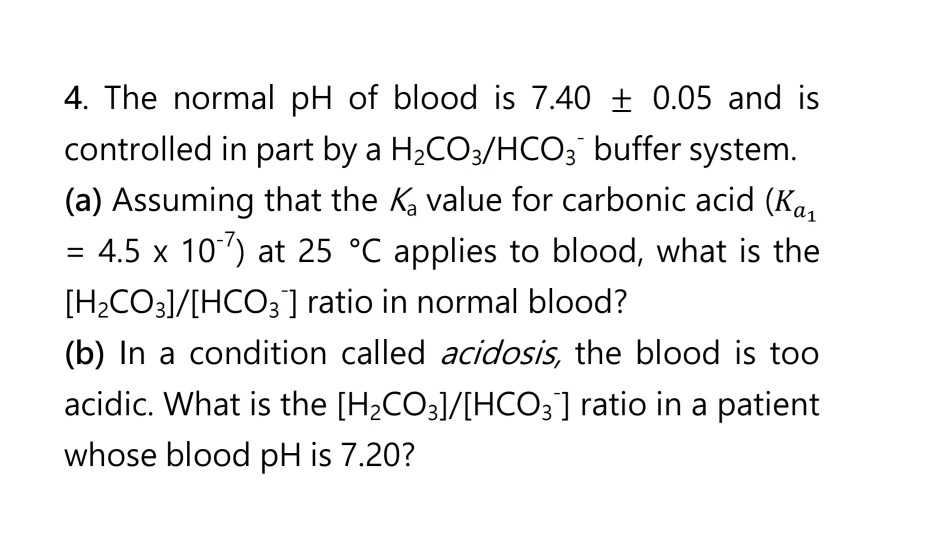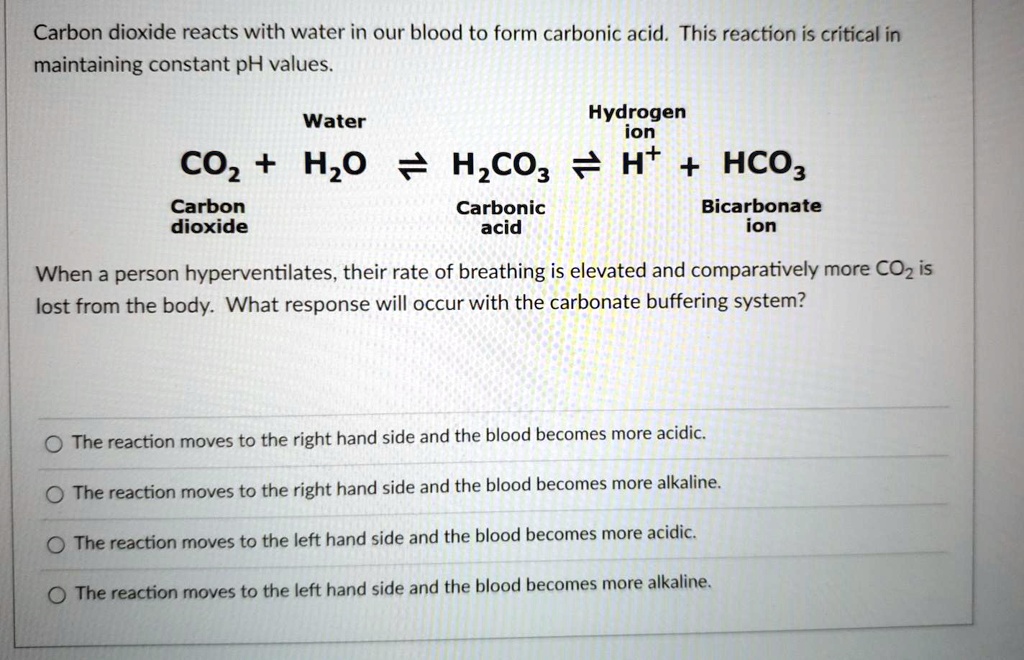
SOLVED: Carbon dioxide reacts with water in our blood to form carbonic acid. This reaction is critical in maintaining constant pH values Water Hydrogen ion HzCOz H+ HCO3 Carbonic Bicarbonate acid ion
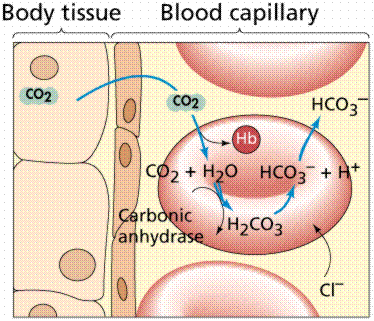
Normally, the pH of the human body is fixed in a very narrow range between 7.35 and 7.45. A patient with an acidotic blood pH of 7.3 may be treated with an