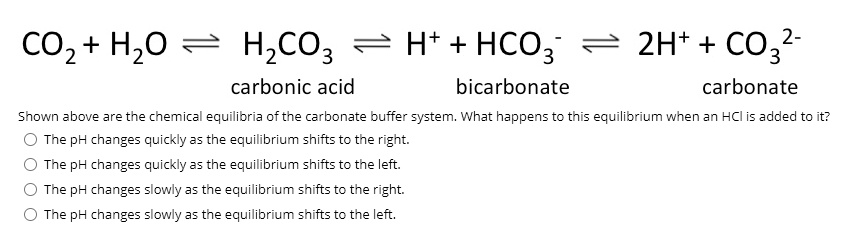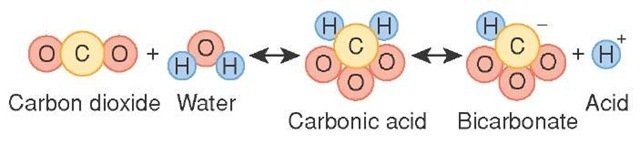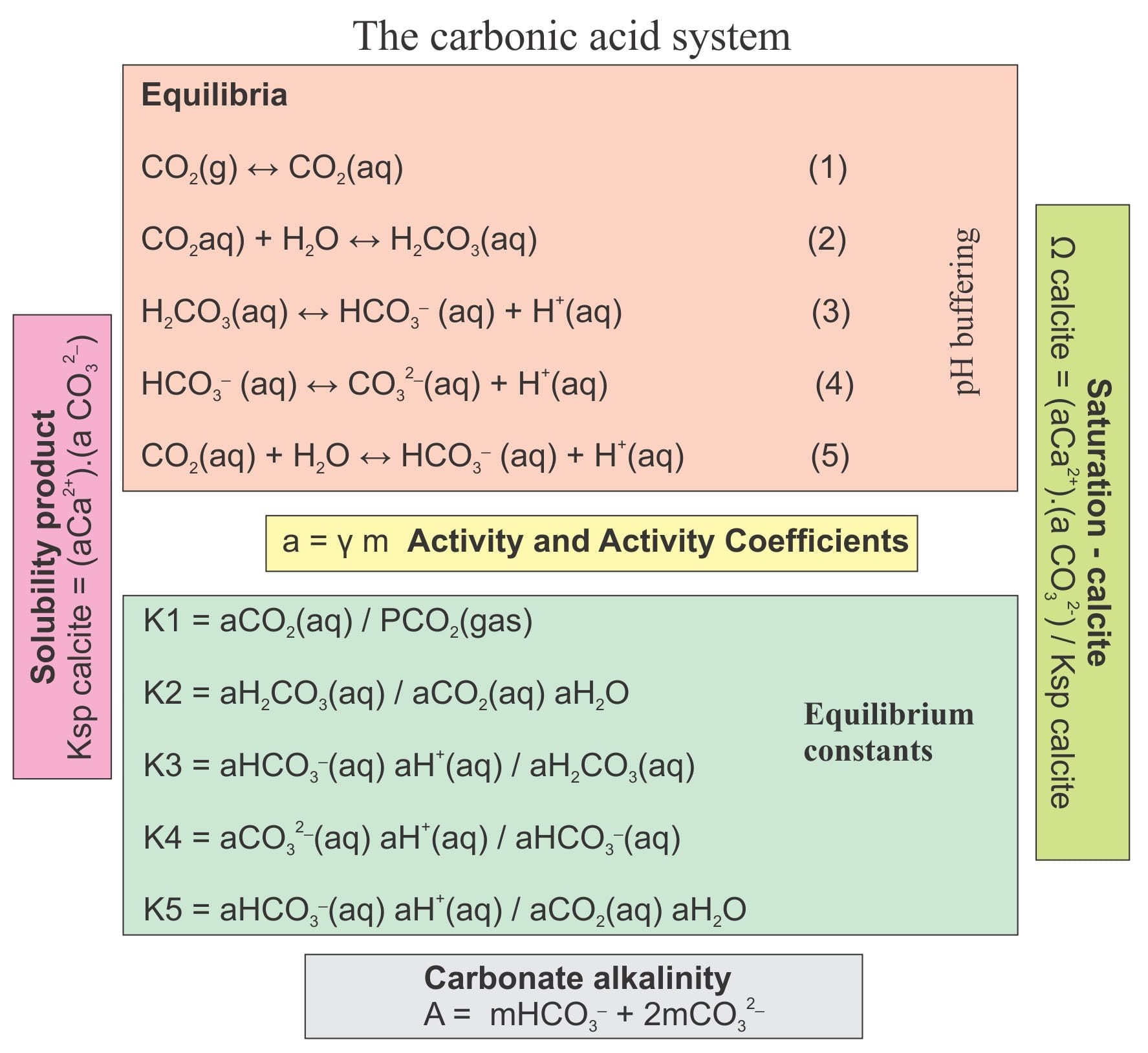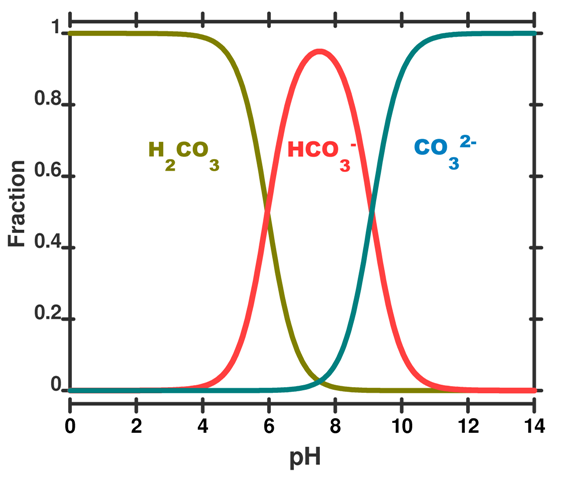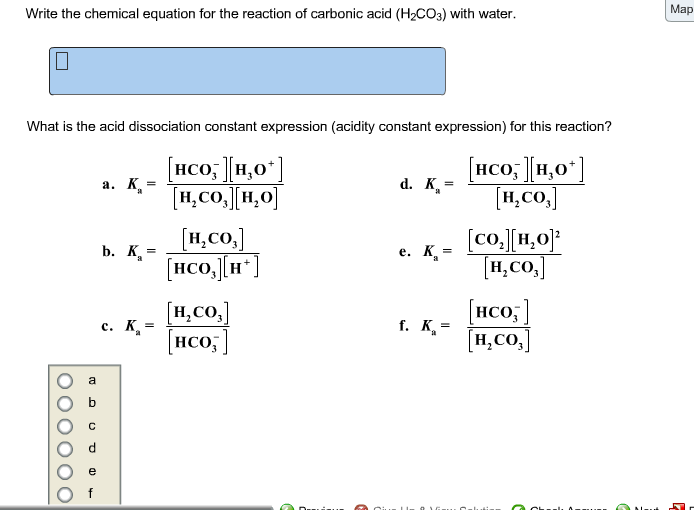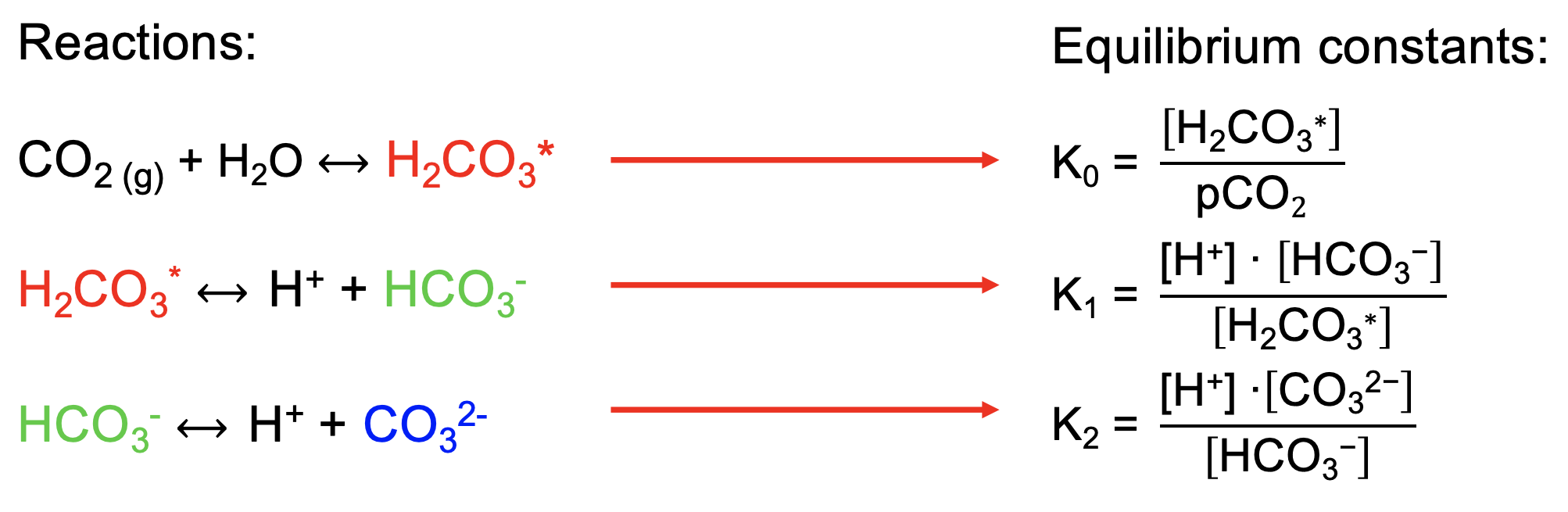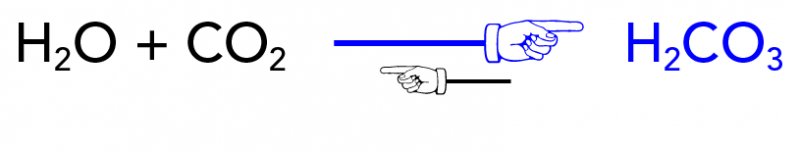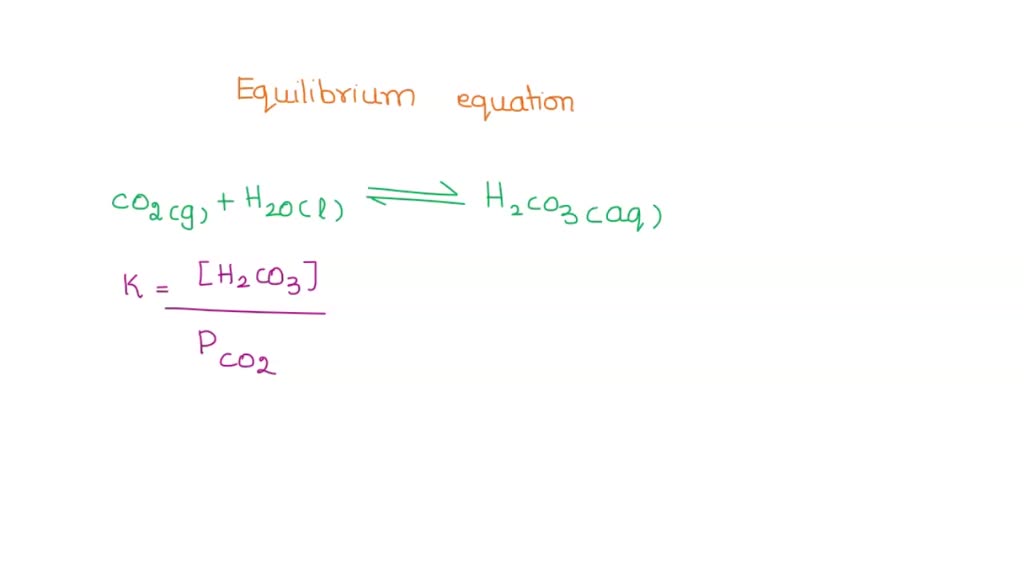
SOLVED: What is the equilibrium equation for the interconversion between carbon dioxide, water, carbonic acid and bicarbonate ions?

OneClass: 14. The equilibrium between carbon dioxide gas and carbonic acid is very im' biology and en...

OneClass: Carbon dioxide dissolves in water to form carbonic acid, which is primarily dissolved CO2. ...
![SOLVED: The equilibrium constant for the second step dissociation of Carbonic Acid could be written as (1 Point) Note Chemical formula of Carbonic Acid is HzCO3 [H- J[co;? LHCO, ] Ka2 [H - SOLVED: The equilibrium constant for the second step dissociation of Carbonic Acid could be written as (1 Point) Note Chemical formula of Carbonic Acid is HzCO3 [H- J[co;? LHCO, ] Ka2 [H -](https://cdn.numerade.com/ask_images/53cbf00864e3490cb82982e4f8e7a63b.jpg)
SOLVED: The equilibrium constant for the second step dissociation of Carbonic Acid could be written as (1 Point) Note Chemical formula of Carbonic Acid is HzCO3 [H- J[co;? LHCO, ] Ka2 [H -

SOLVED: The equilibrium between carbon dioxide gas and carbonic acid is very important in biology and environmental science. CO2 (aq) + H2O (l) H2CO3 (aq) Which one of the following is the