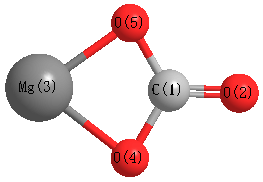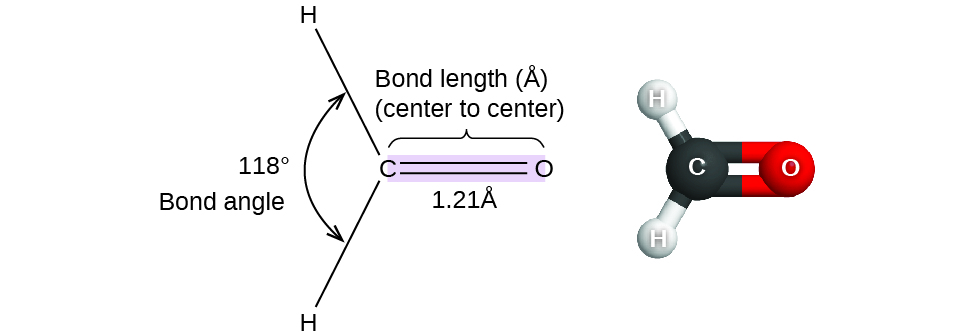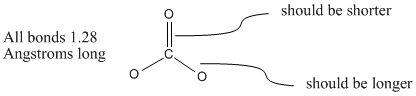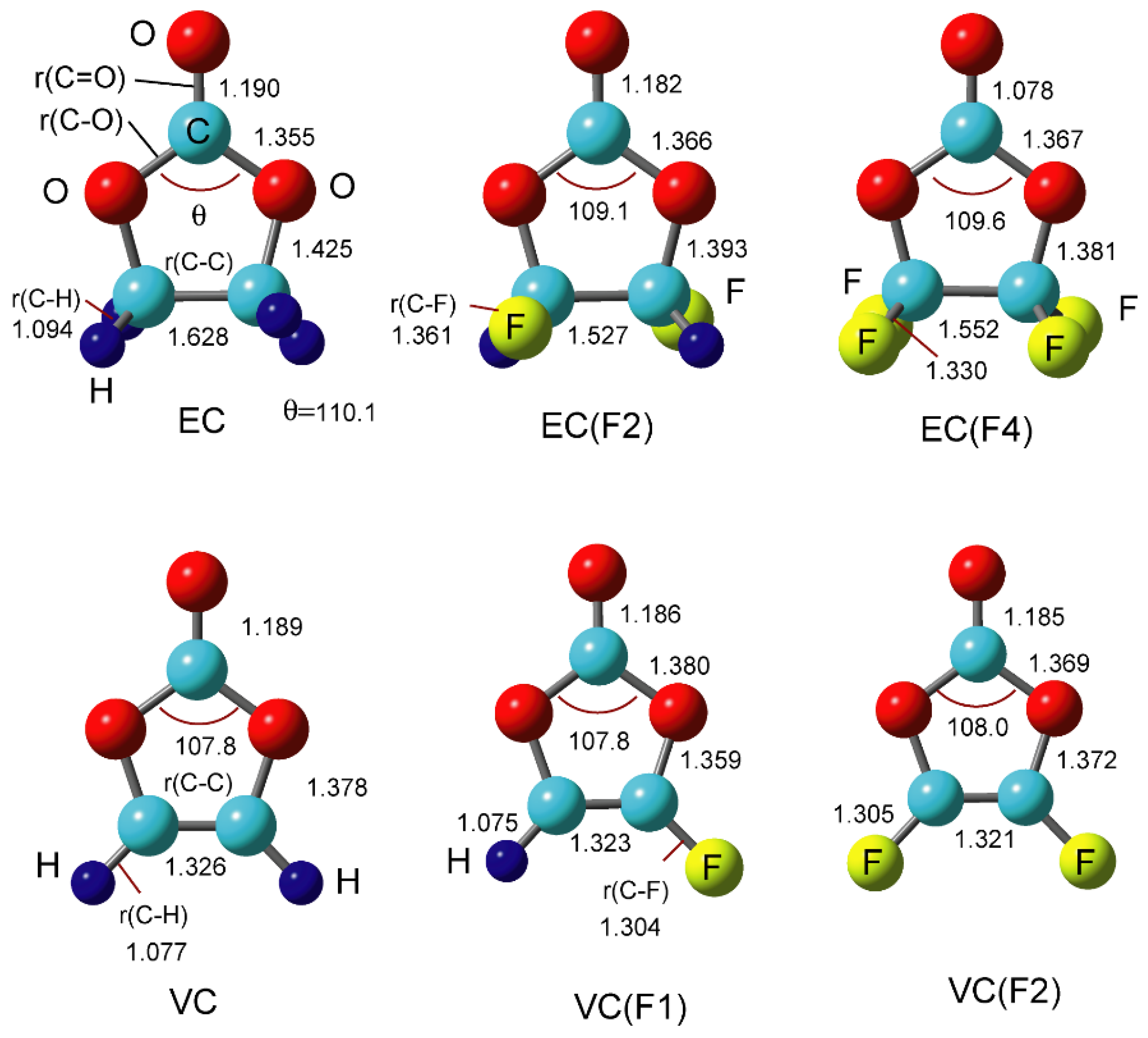
Atoms | Free Full-Text | Density Functional Theory (DFT) Study on the Ternary Interaction System of the Fluorinated Ethylene Carbonate, Li+ and Graphene Model

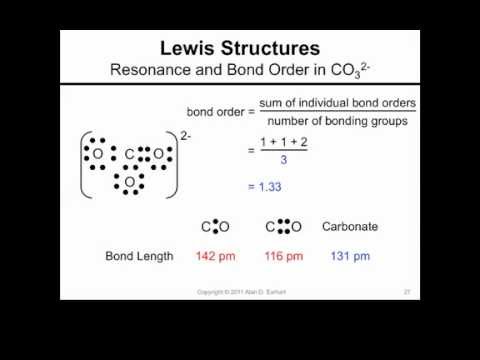
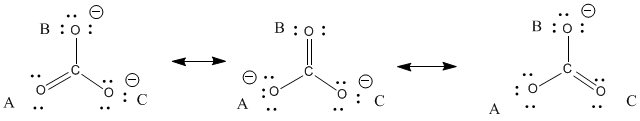
Chapter 6 Resonance and Electron Delocalization. Chapter 6 Topics for Test u Sections 6.1 through 6.13 u I will emphasize drawing resonance structures-- - ppt download
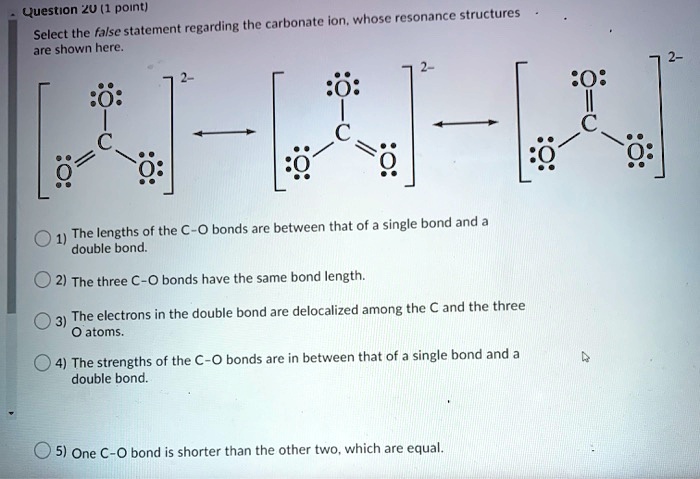
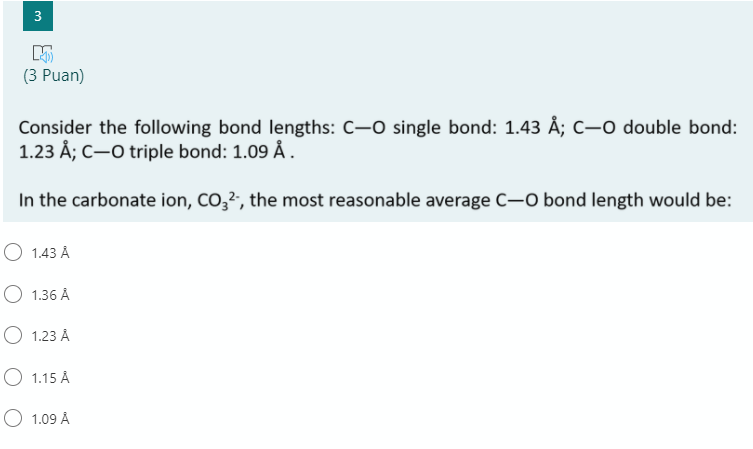
SOLVED: Question *2U (1 point) the carbonate ion, whose resonance structures Select the false statement regarding are shown here 0 bonds arc between that of single bond and The lengths of the
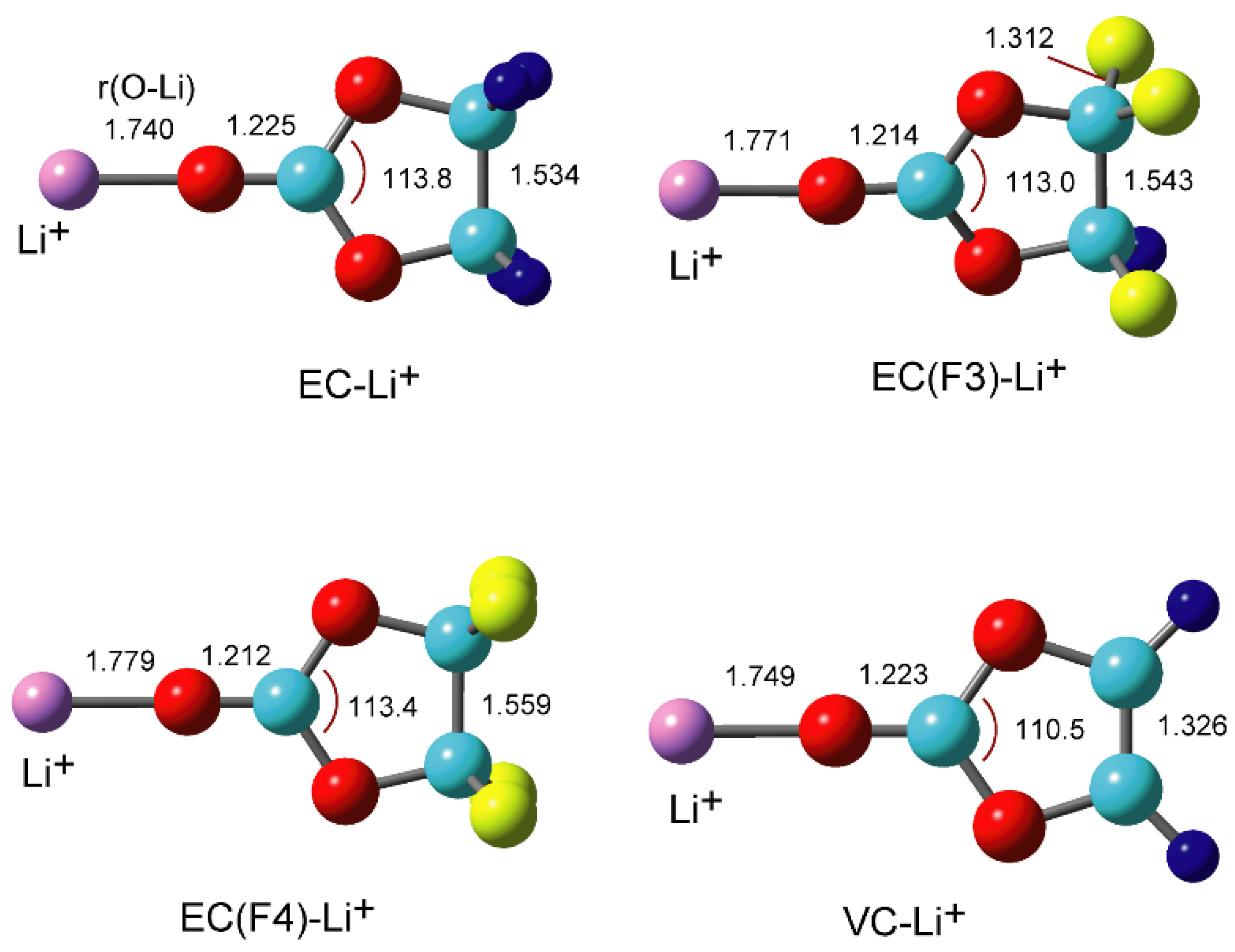
Atoms | Free Full-Text | Density Functional Theory (DFT) Study on the Ternary Interaction System of the Fluorinated Ethylene Carbonate, Li+ and Graphene Model