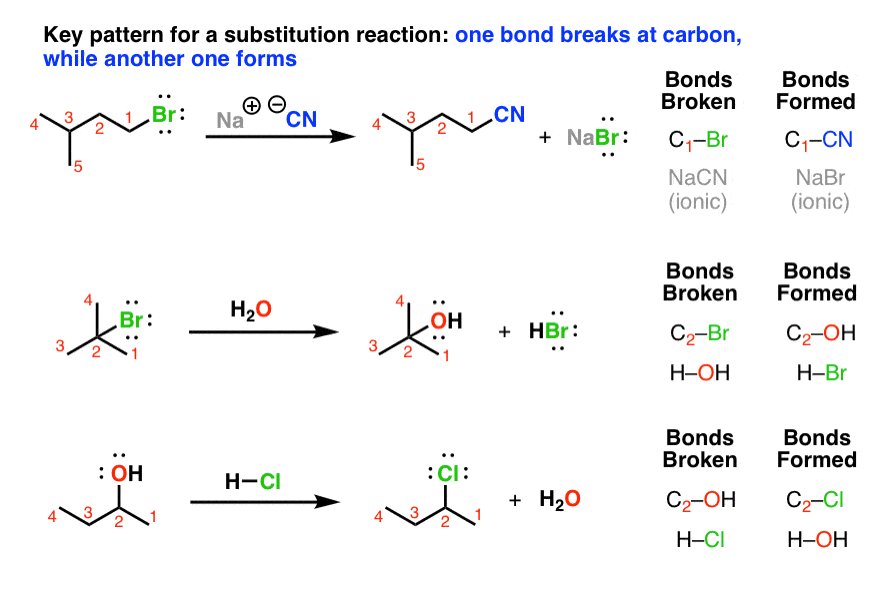
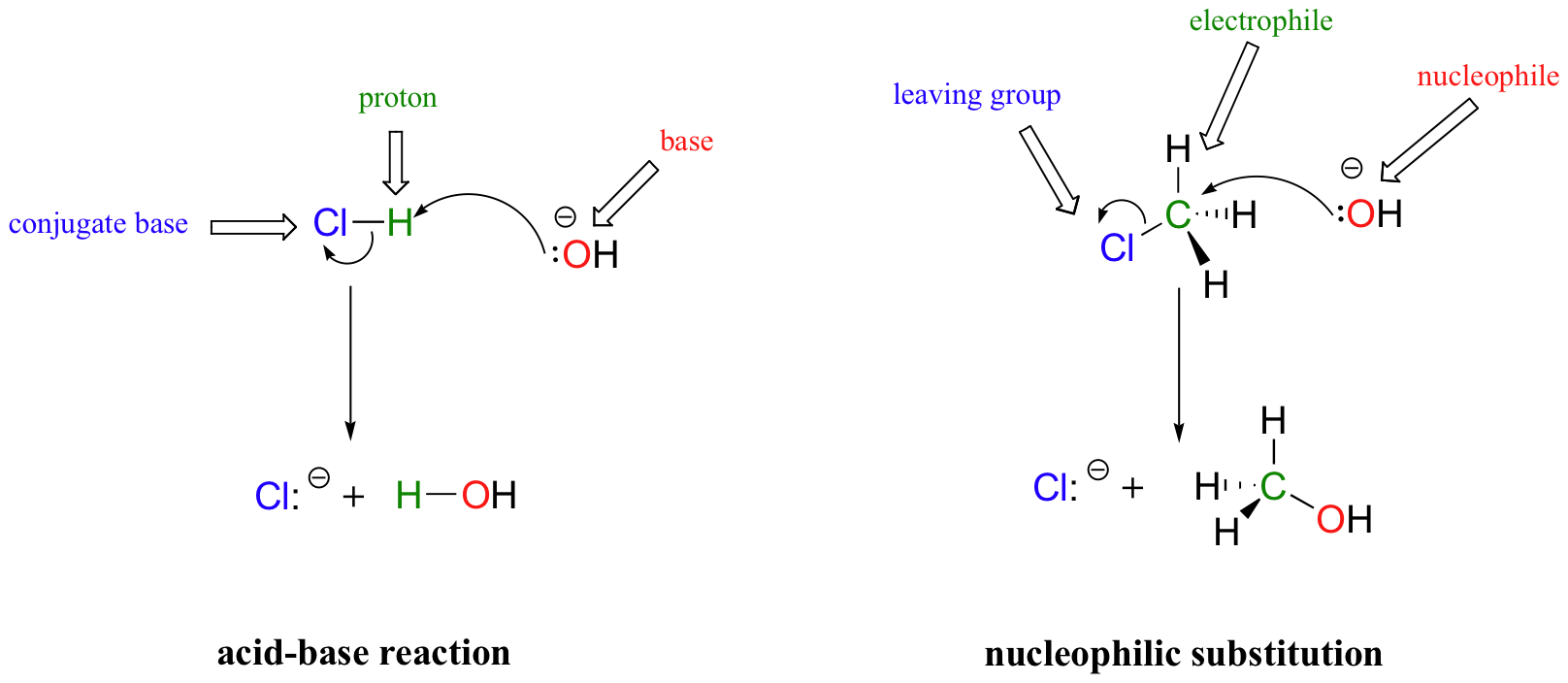
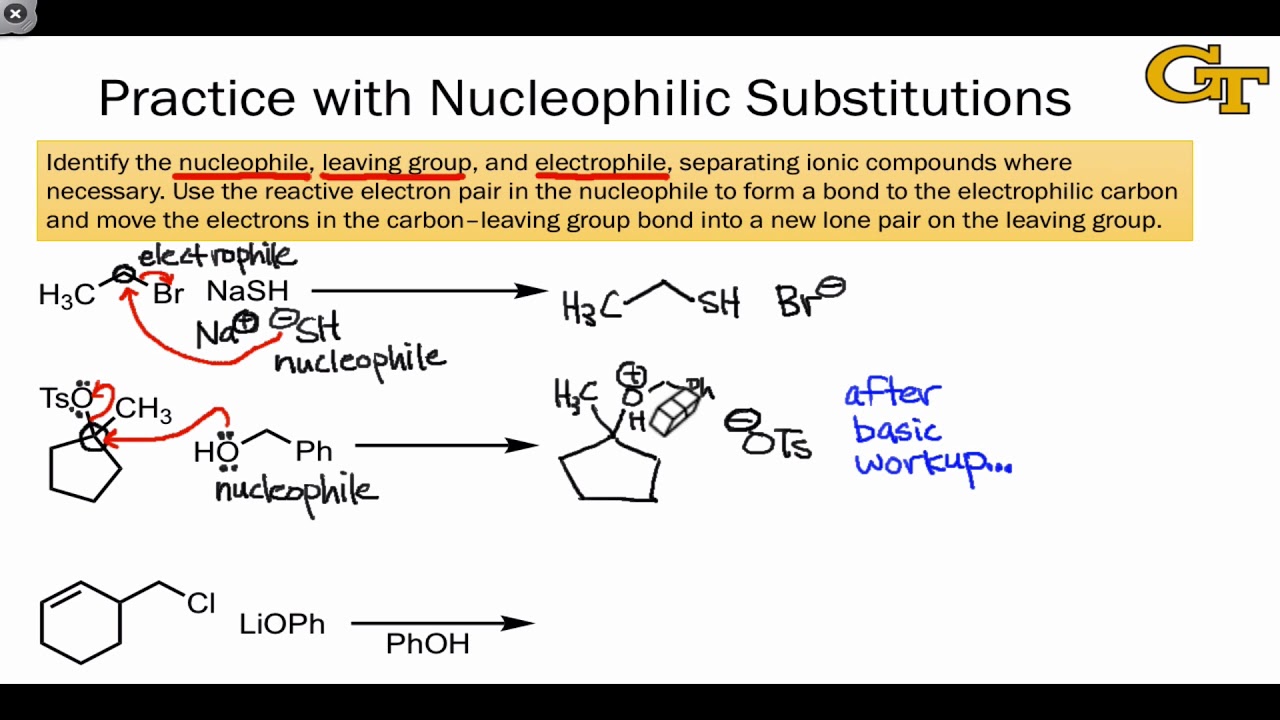
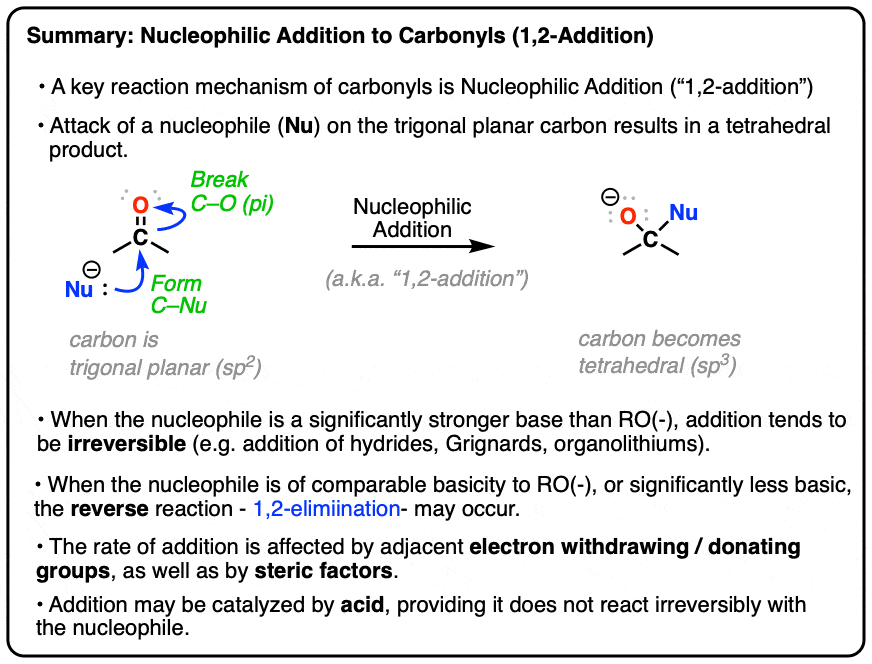
Nucleophilic Substitution at the Guanidine Carbon Center via Guanidine Cyclic Diimide Activation | Organic Letters
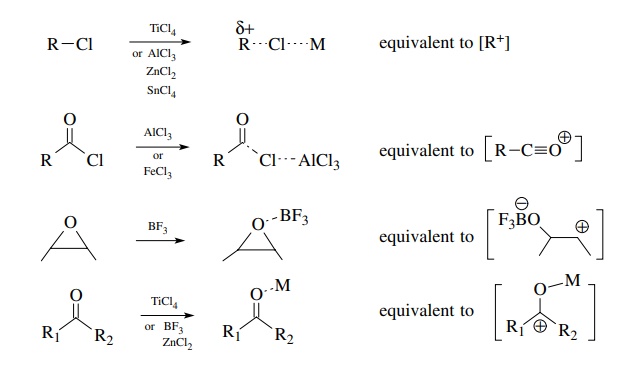
Neutral Carbon Nucleophiles - Carbon-Carbon Bond Formation Between Carbon Nucleophiles and Carbon Electrophiles | Organic Chemistry

Mild C–C Bond Formation via Lewis Acid Catalyzed Oxetane Ring Opening with Soft Carbon Nucleophiles - Huang - 2021 - Angewandte Chemie International Edition - Wiley Online Library

Mechanochemical synthesis of magnesium-based carbon nucleophiles in air and their use in organic synthesis | Nature Communications

Michael Addition of Soft Carbon Nucleophiles to Alkylidene Isoxazol-5-ones: A Divergent Entry to β-Branched Carbonyl Compounds. | Semantic Scholar

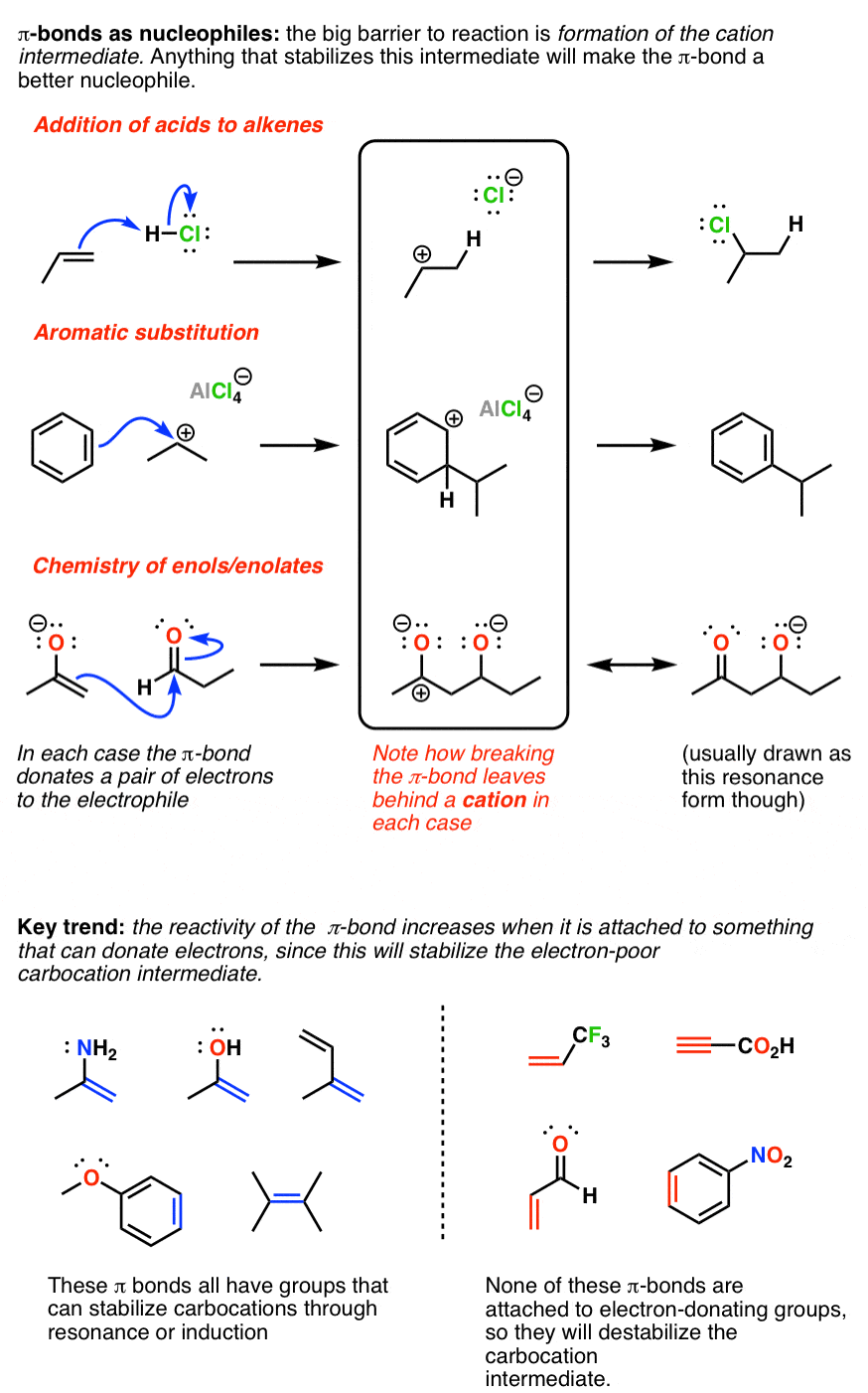

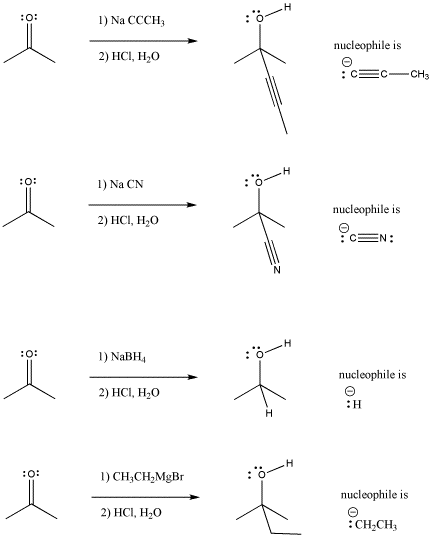
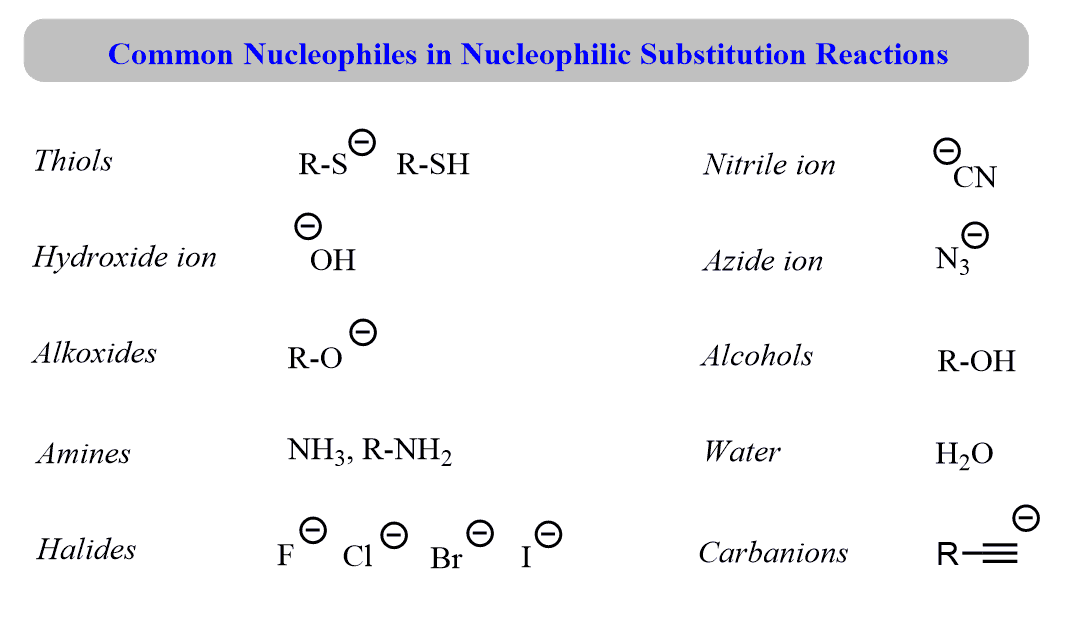
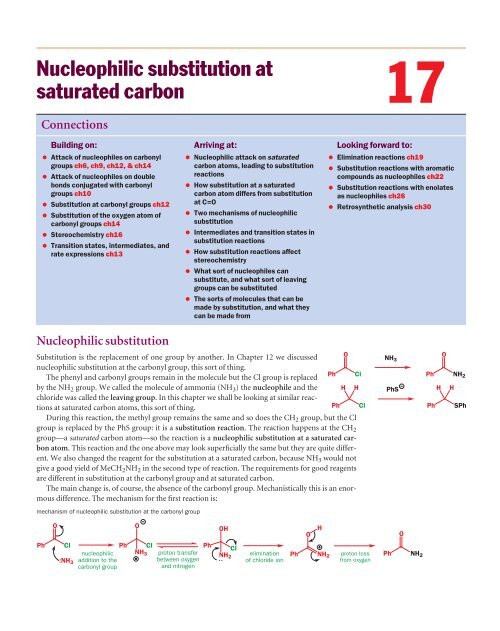
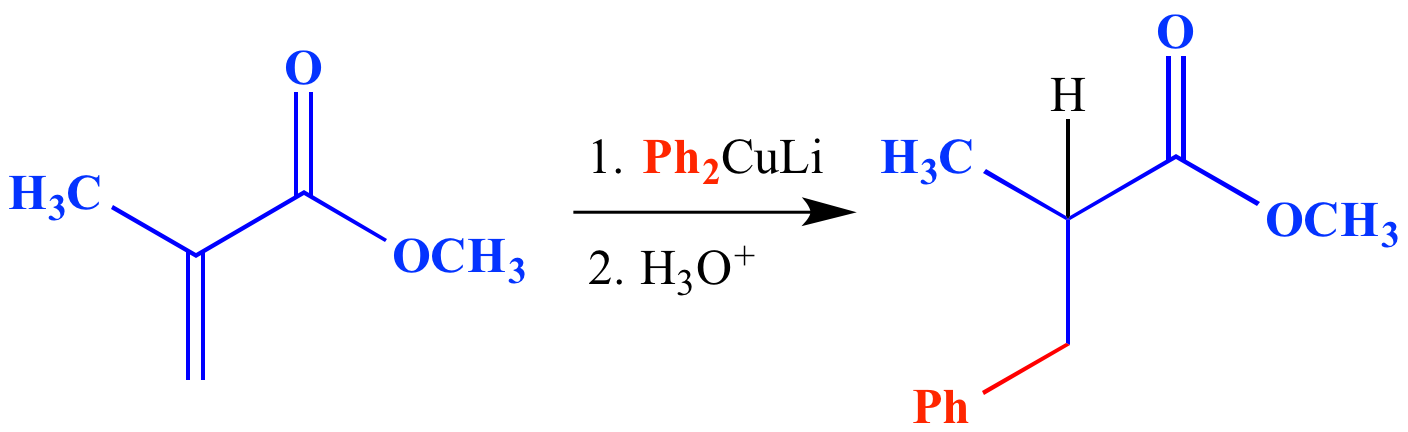
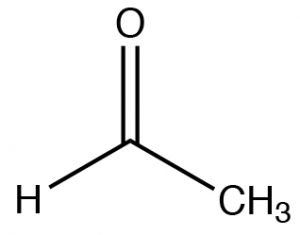
/chapter6/pages45and46/page45and46_files/hardsoftelec.png)