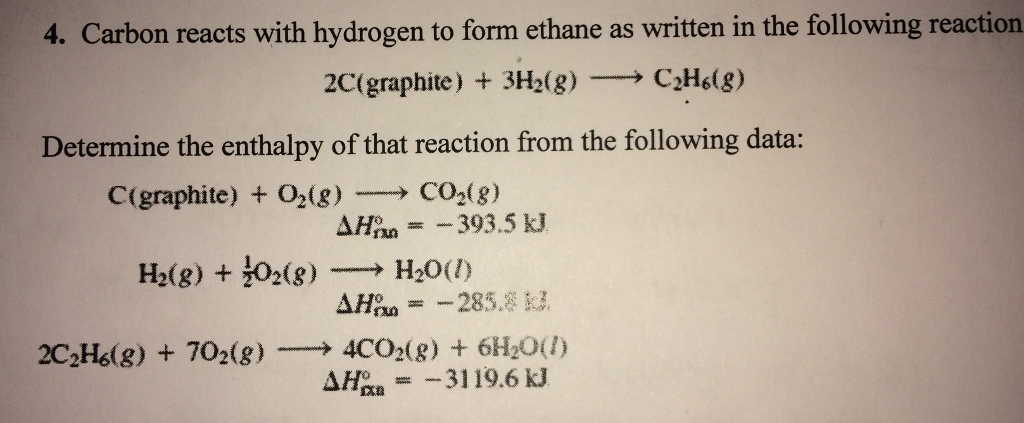Carbon Monoxide as a Promoter of Atomically Dispersed Platinum Catalyst in Electrochemical Hydrogen Evolution Reaction | Journal of the American Chemical Society

Understanding the kinetics of carbon-hydrogen reaction: Insights from reaction mechanisms on zigzag edges for homogeneous and heterogeneous formation of methane - ScienceDirect
![The molecule below contains carbon atoms (shown in black), hydrogen (white), and oxygen (red). [{Image src='reaction8141246017280565756.jpg' alt='reaction' caption=''}] | Homework.Study.com The molecule below contains carbon atoms (shown in black), hydrogen (white), and oxygen (red). [{Image src='reaction8141246017280565756.jpg' alt='reaction' caption=''}] | Homework.Study.com](https://homework.study.com/cimages/multimages/16/reaction8141246017280565756.jpg)
The molecule below contains carbon atoms (shown in black), hydrogen (white), and oxygen (red). [{Image src='reaction8141246017280565756.jpg' alt='reaction' caption=''}] | Homework.Study.com

Carbon–Hydrogen versus Nitrogen–Oxygen Bond Activation in Reactions of N-Oxide Derivatives of 2,2′-Bipyridine and 1,10-Phenanthroline with a Dimethylplatinum(II) Complex | Organometallics
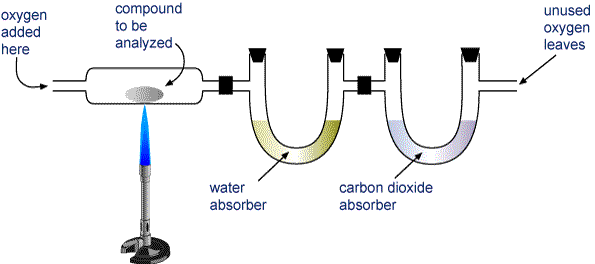
In the combustion analysis of an unknown compound containing only carbon , hydrogen and oxygen, the grams of oxygen are found from the grams of? A) CO2 and unknown compound B) CO2,H2O

hydrocarbons - Why carbon monoxide reacts with hydrogen to give different products in the presence of different catalysts - Chemistry Stack Exchange
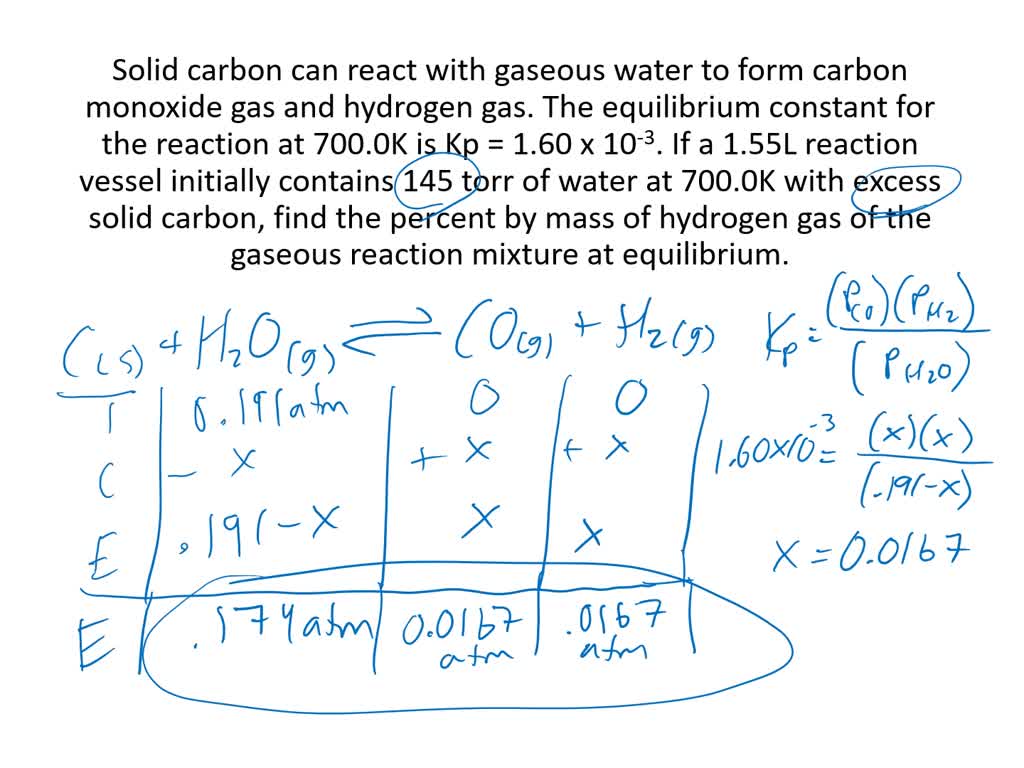
SOLVED:Solid carbon can react with gaseous water to form carbon monoxide gas and hydrogen gas. The equilabrium constant for the reaction at 700.0 K is Kp-1.60 ×10^-3. If a 1.55-L. reaction vessel

Carbon monoxide reacts with hydrogen under certain conditions to from methanol `(CH_(3)OH)` - YouTube

Understanding the kinetics of carbon-hydrogen reaction: Insights from reaction mechanisms on zigzag edges for homogeneous and heterogeneous formation of methane - ScienceDirect

Molybdenum‐Based Carbon Hybrid Materials to Enhance the Hydrogen Evolution Reaction - Bae - 2018 - Chemistry – A European Journal - Wiley Online Library

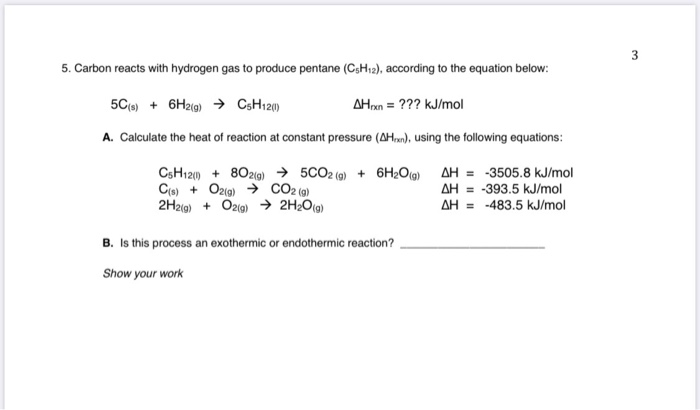
The combustion enthalpies of carbon, hydrogen and ethyne are - 393.5 kJ mol^-1, - 285.8 kJ mol^-1 and - 1309.5 kJ mol^-1 respectively at 25^oC . The value of standard enthalpy of


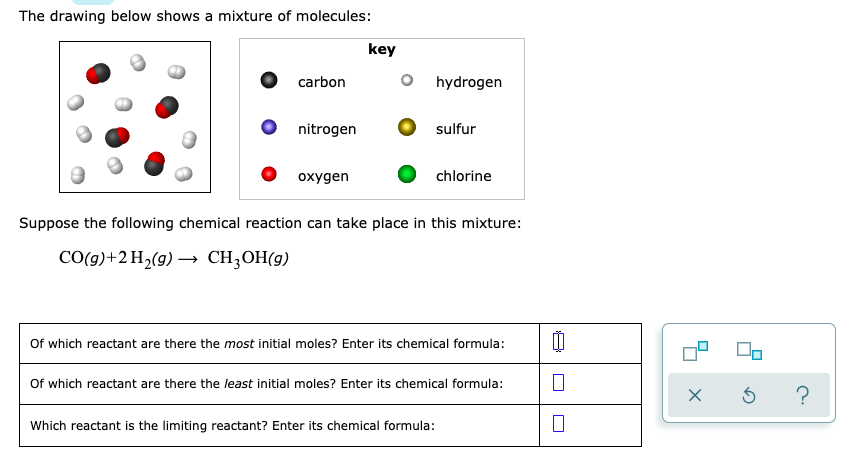

.jpg)