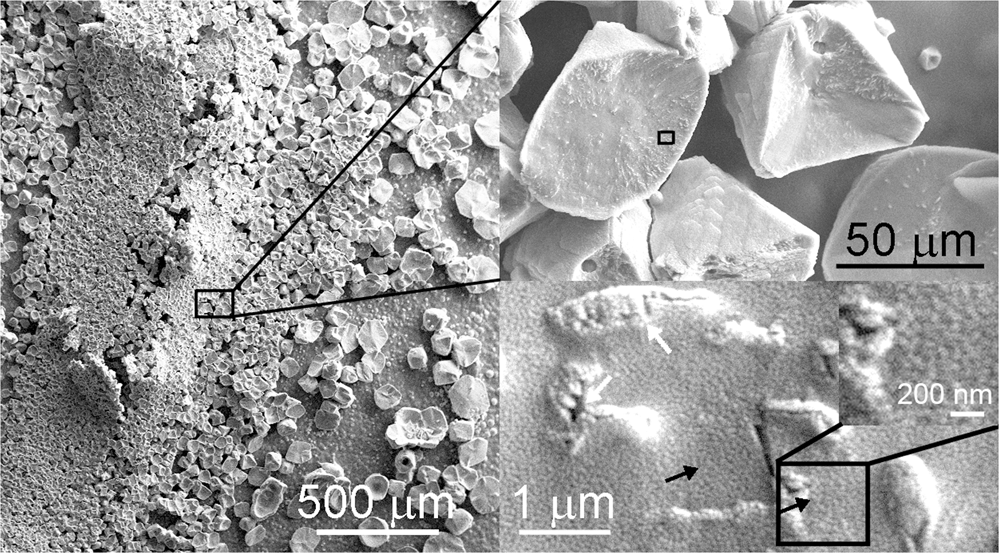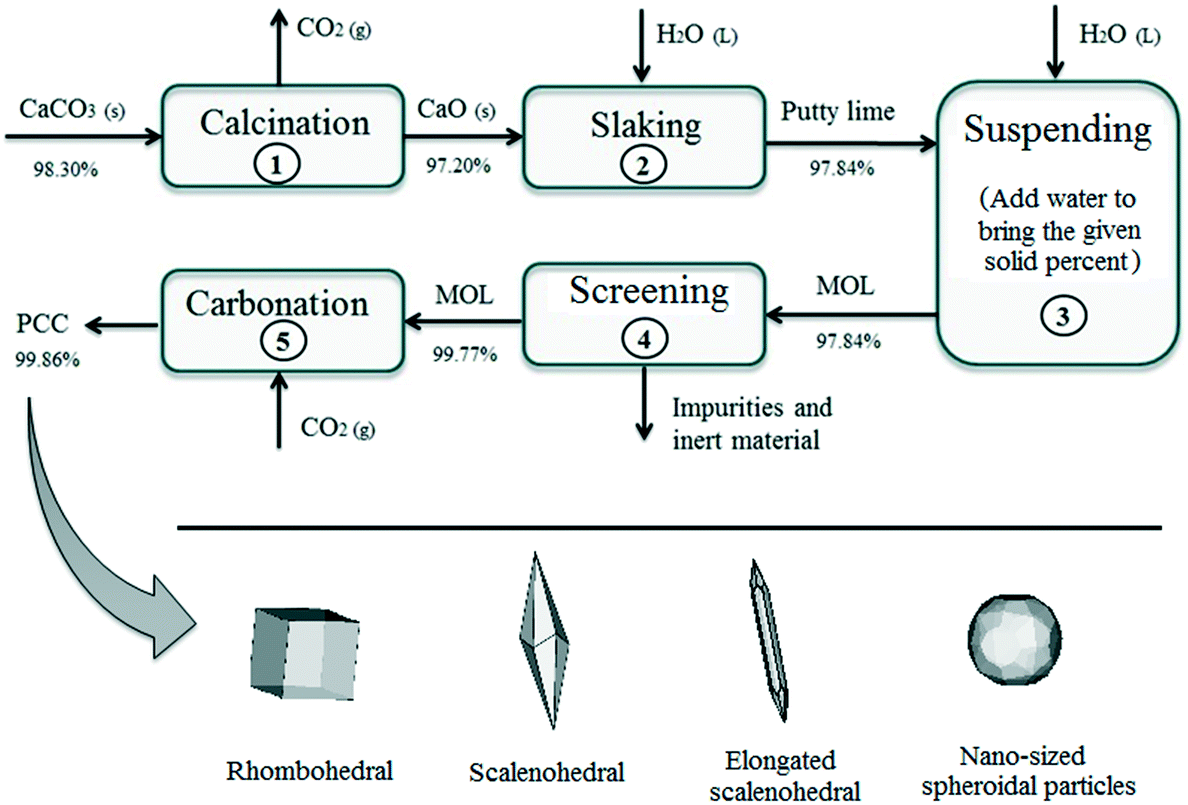
Control of the morphology, specific surface area and agglomeration of precipitated calcium carbonate crystals through a multiphase carbonation process ... - CrystEngComm (RSC Publishing) DOI:10.1039/C9CE01876J

Urease-aided calcium carbonate mineralization for engineering applications: A review - ScienceDirect

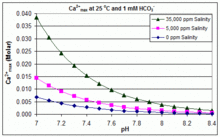
Effect of pH and Phosphate on Calcium Carbonate Polymorphs Precipitated at near-Freezing Temperature | Crystal Growth & Design

Materials | Free Full-Text | Precipitation and Transformation of Vaterite Calcium Carbonate in the Presence of Some Organic Solvents
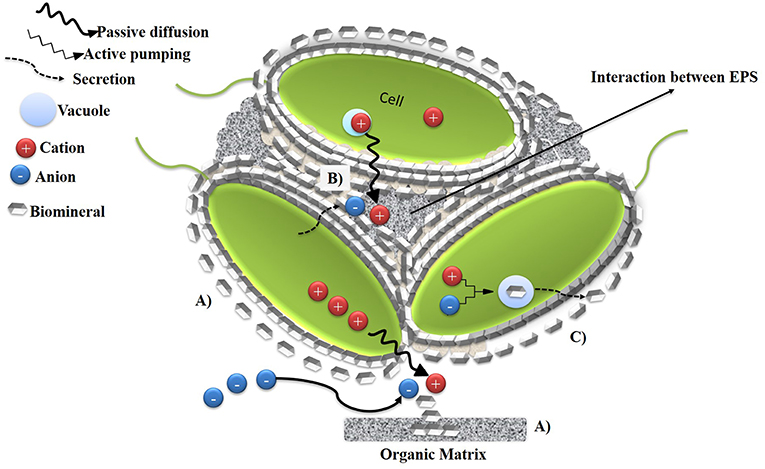
Frontiers | Microbially Induced Calcium Carbonate Precipitation (MICP) and Its Potential in Bioconcrete: Microbiological and Molecular Concepts
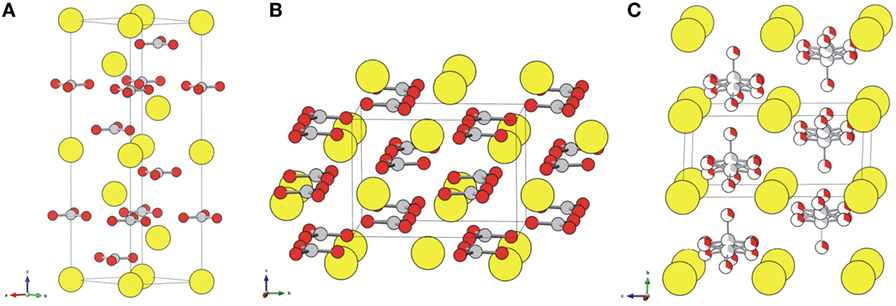
Frontiers | Calcium Carbonate Precipitation for CO2 Storage and Utilization: A Review of the Carbonate Crystallization and Polymorphism