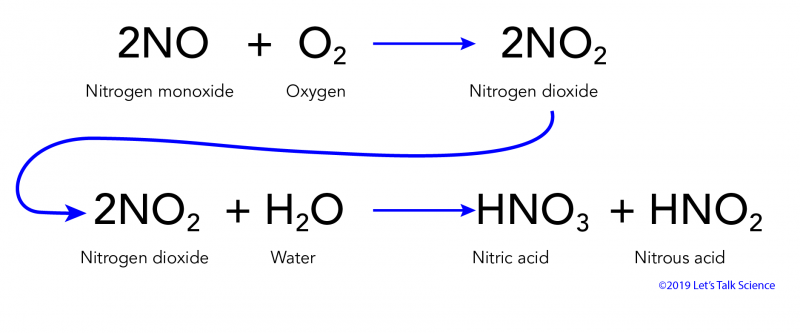
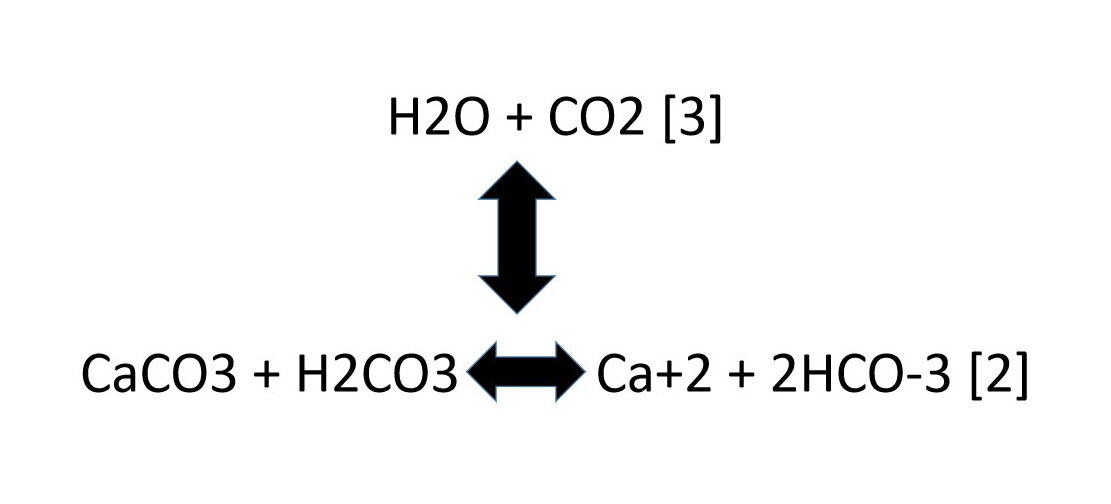
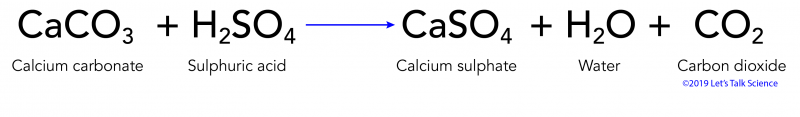
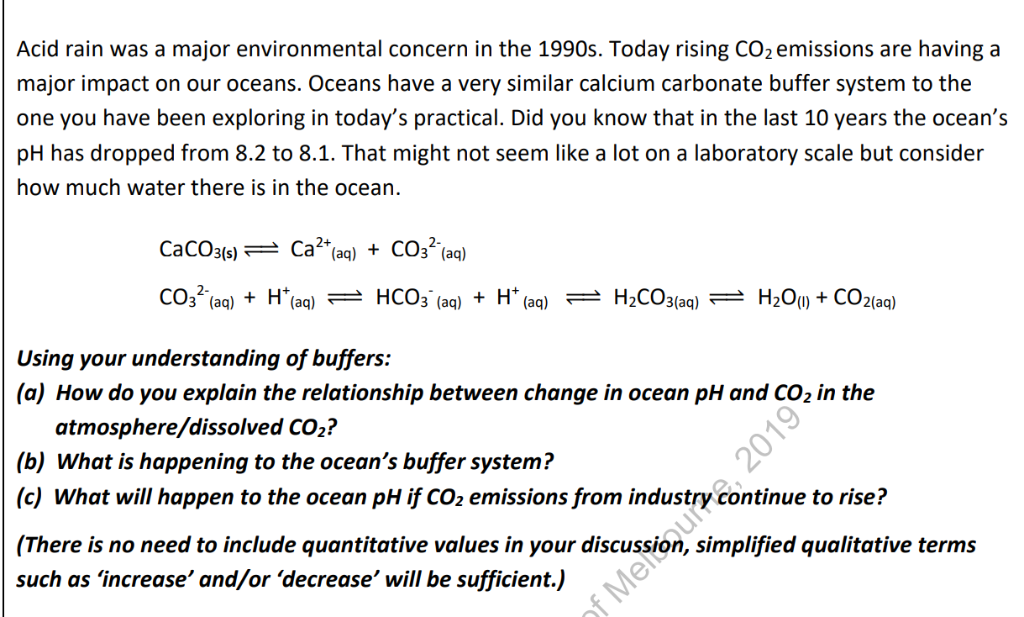
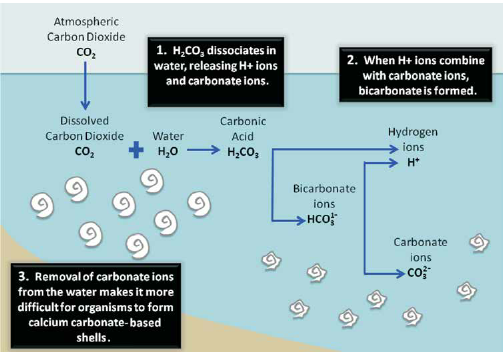
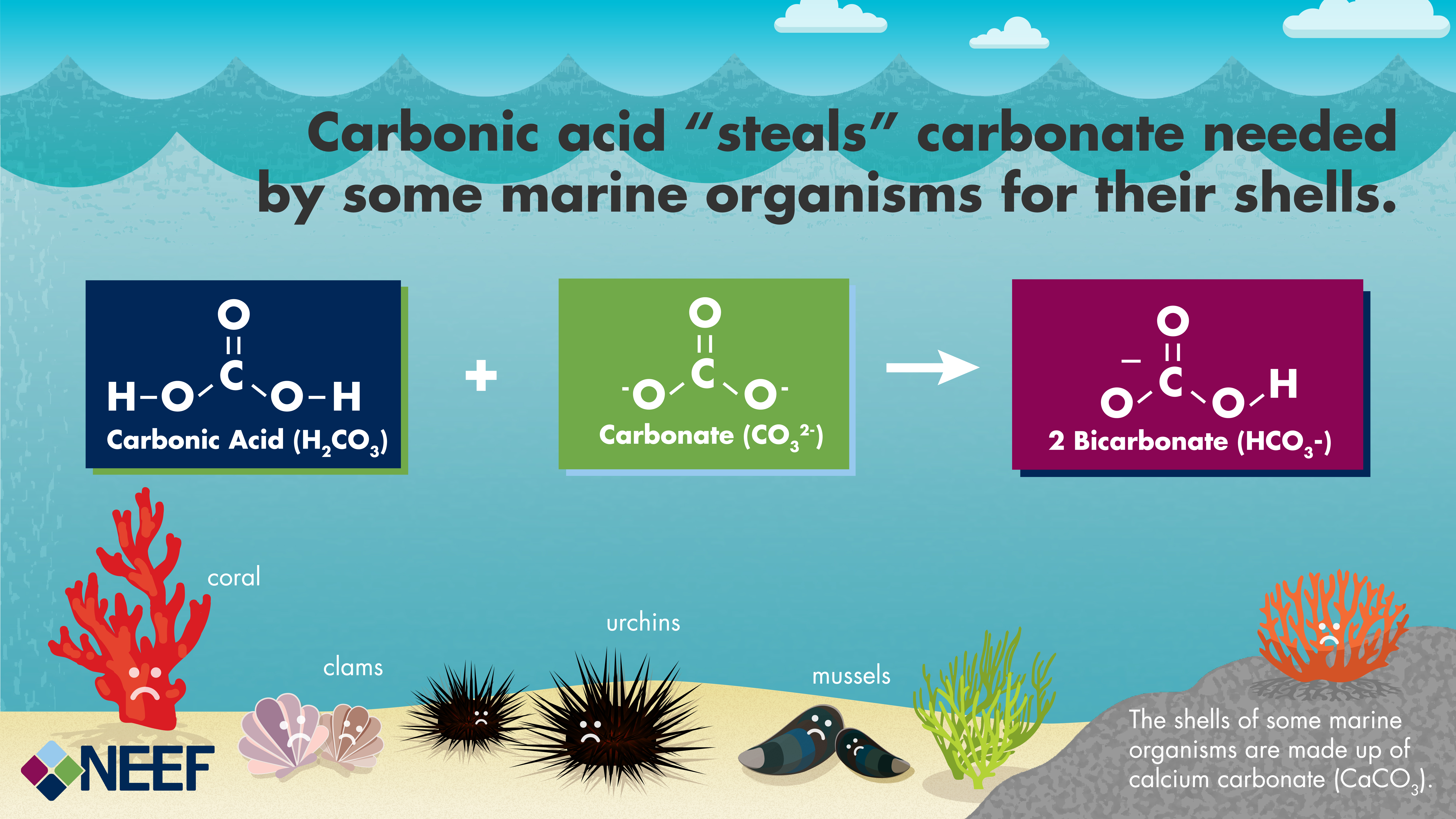
Acids, Bases and Salts Acids give up hydrogen ions (H+) in a water solution. Bases give up hydroxide ions (OH-) in a water solution. Mullis. - ppt video online download
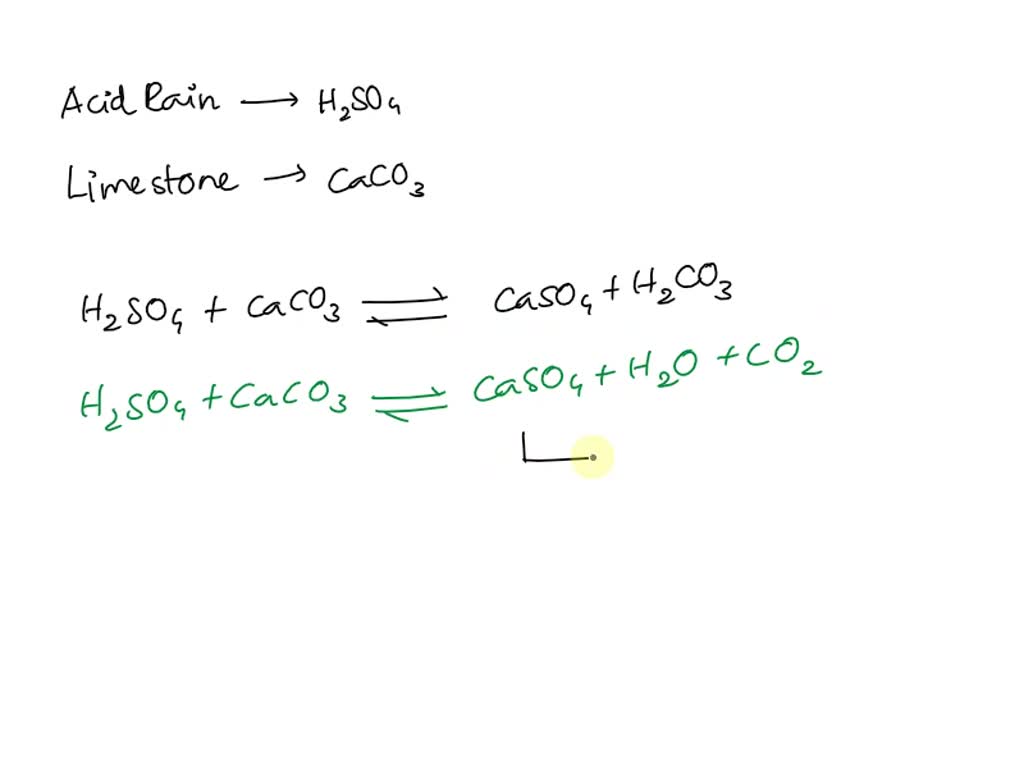
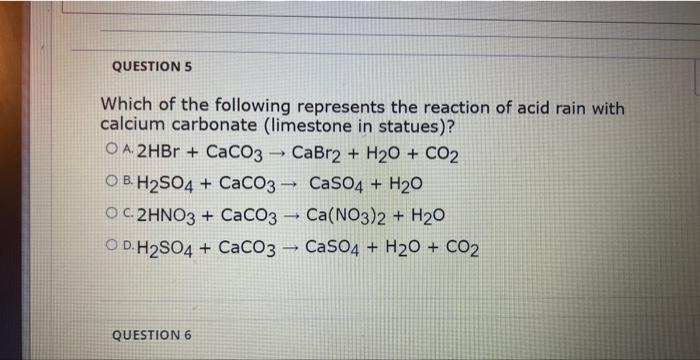
SOLVED: Acid rain is a dilute solution of acids that dissolve the calcium carbonate in limestone statues. Concentrated acids can dissolve a large piece of limestone in a few days. Statue breakdown

Effect of simulated acid rain on the stability of calcium carbonate immobilized by microbial carbonate precipitation - ScienceDirect
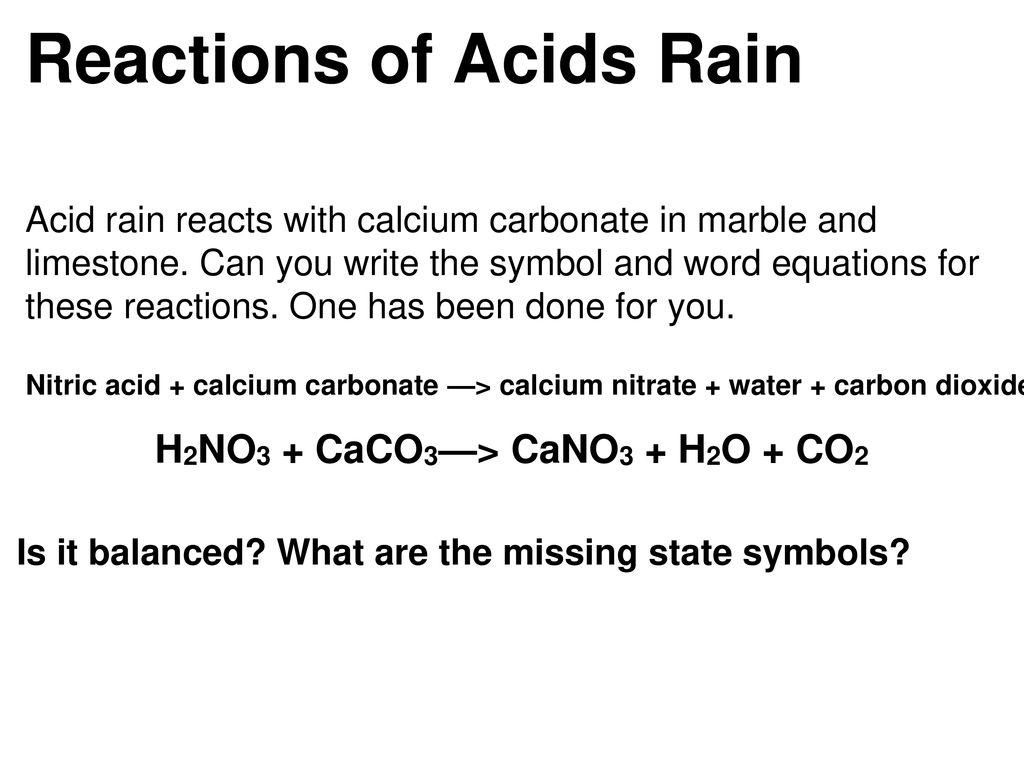
CWK CWK Acid Rain State the adverse effect of these common pollutants on buildings and why these pollutants are of global concern Relate the effects. - ppt download

Acid Deposition Lake Barkevatn in Norway used to have healthy stocks of trout and perch. As a result of acid rain, the trout stock died out in the mid-1970s. - ppt download

Toward the Mechanistic Understanding of the Additives' Role on Ammonium Nitrate Decomposition: Calcium Carbonate and Calcium Sulfate as Case Studies | ACS Omega

Everyday acid and base reactions. Calcium carbonate and rocks. Limestone is also largely composed of calcium carbonate. Bath Stone (Greater Oolite) is. - ppt download