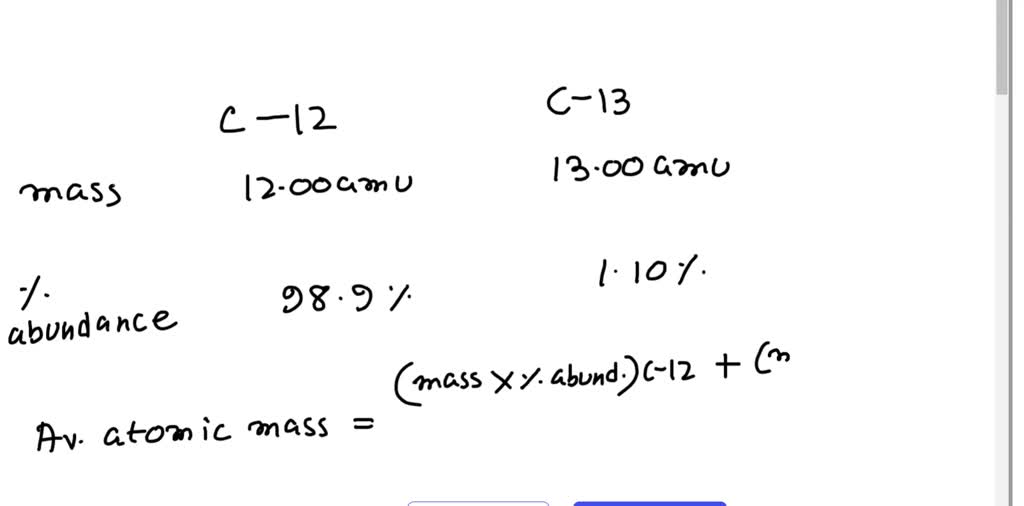1.4 Isotopes, Radioisotopes, and Atomic Mass B3.1 explain the relationship between the atomic number and the mass number of an element, and the difference. - ppt download

Draw the circular diagram to represent the atomic mass of carbon-12for one atomic mass unit - Brainly.in

SOLVED: An isotope of nitrogen is found to have an atomic mass that is 1.25001 times the atomic mass of carbon-12. What is the atomic mass of this isotope? amu 2) The

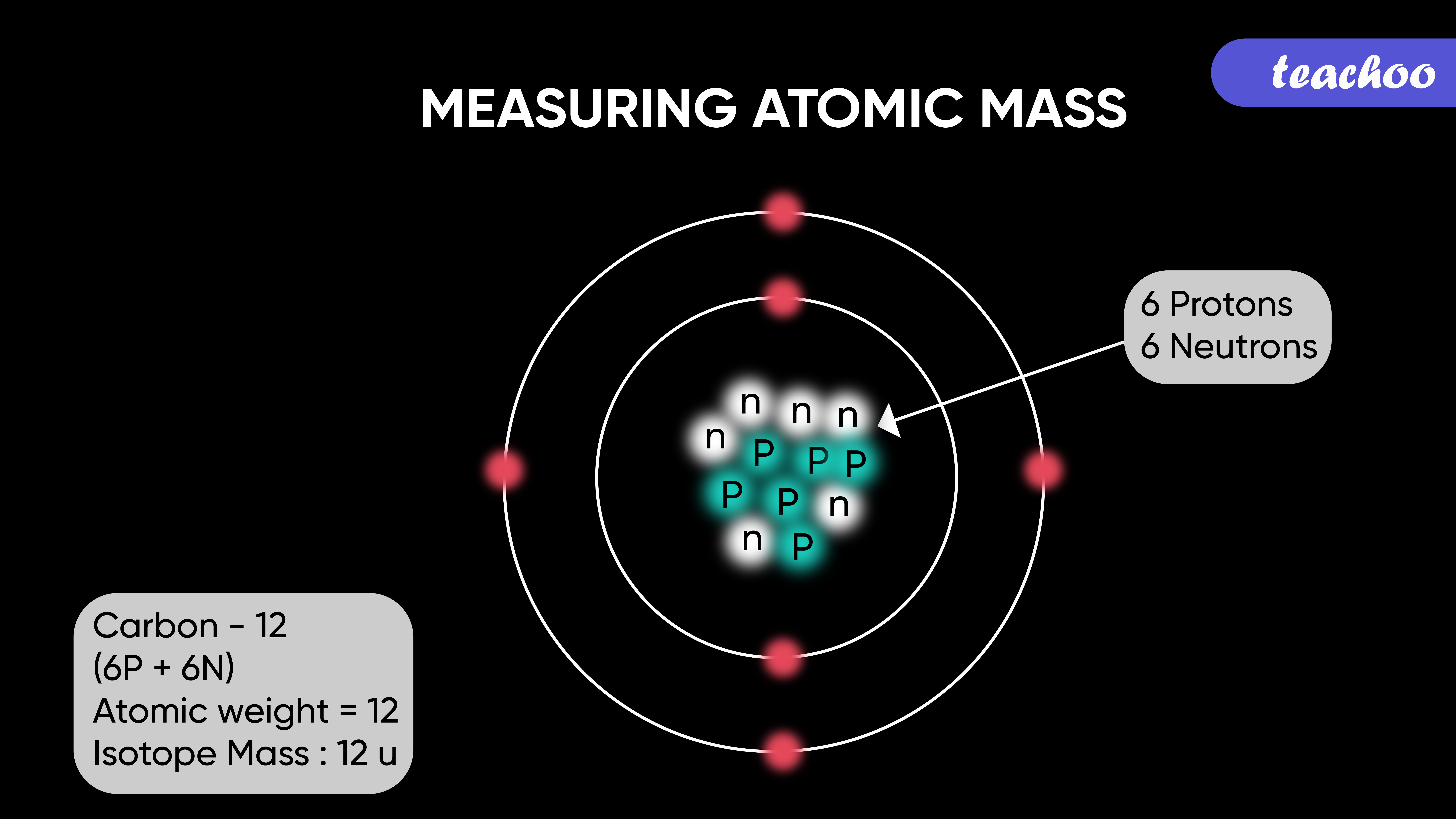
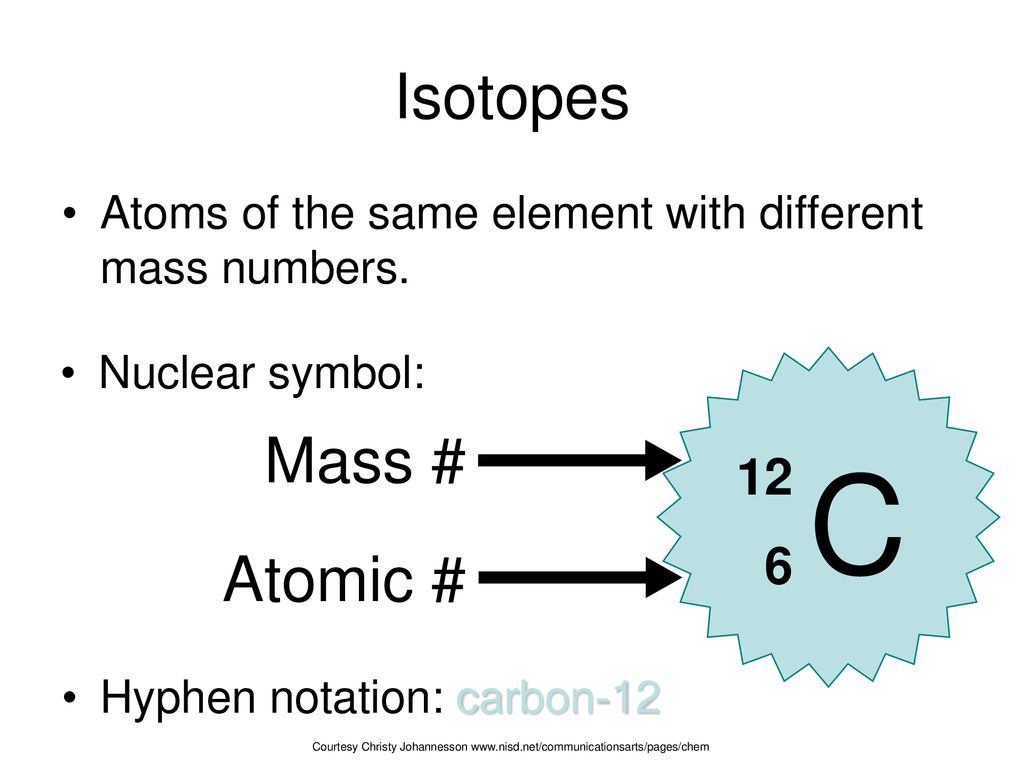
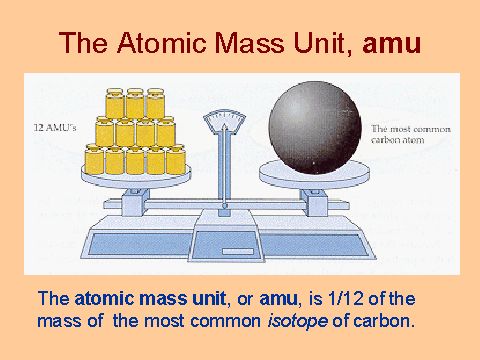
Atomic Mass Unit: amu (atomic mass unit) amu is defined as a mass exactly equal to on-twelfth the mass of Carbon-12 atom amu = 1/12 of carbon-12 Hydrogen. - ppt download

Katie Mummah on Twitter: "So the atomic mass has to take into account these isotopes. Each of the individual atomic masses is weighted by it's natural abundance to get the atomic weight

Atomic Mass Standard mass unit is derived from carbon 12 Atomic mass unit – the mass equal to 1/12 the mass of one Carbon 12 atom. - ppt download
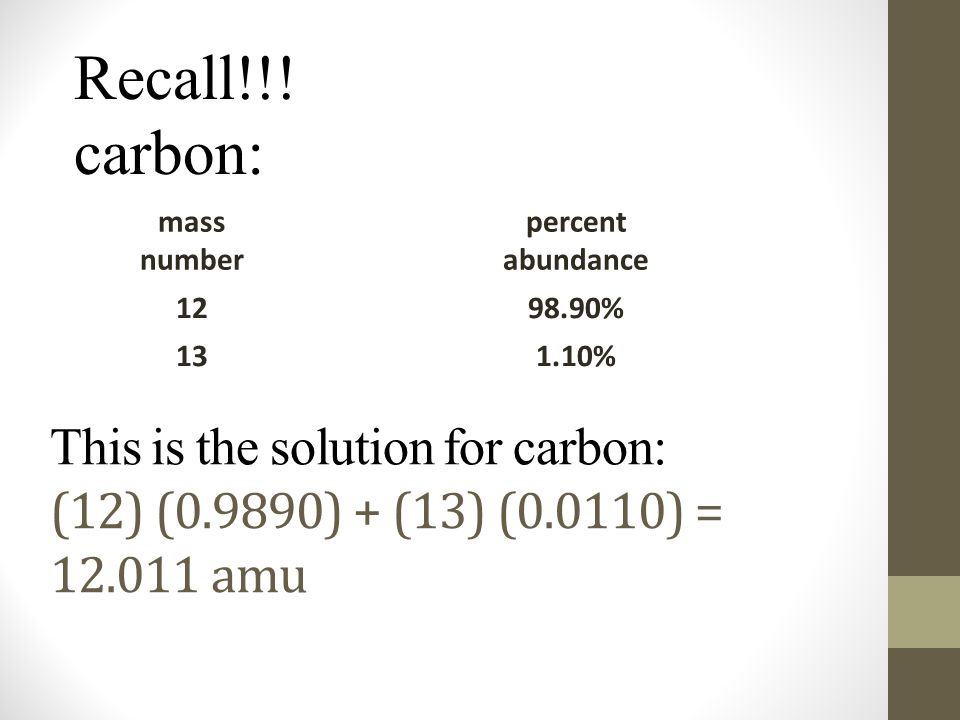
This is the solution for carbon: (12) (0.9890) + (13) (0.0110) = amu mass number percent abundance % % Recall!!! carbon: - ppt download